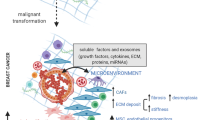
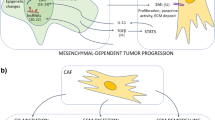
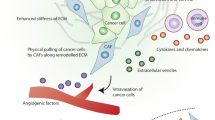
Abstract
Tumor cells exist in close proximity with non-malignant cells. Extensive and multilayered crosstalk between tumor cells and stromal cells tailors the tumor microenvironment (TME) to support survival, growth, and metastasis. Fibroblasts are one of the largest populations of non-malignant host cells that can be found within the TME of breast, pancreatic, and prostate tumors. Substantial scientific evidence has shown that these cancer-associated fibroblasts (CAFs) are not only associated with tumors by proximity but are also actively recruited to developing tumors where they can influence other cells of the TME as well as influencing tumor cell survival and metastasis. This review discusses the impact of CAFs on breast cancer biology and highlights their heterogeneity, origin and their role in tumor progression, ECM remodeling, therapy resistance, metastasis, and the challenges ahead of targeting CAFs to improve therapy response.



Similar content being viewed by others
References
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144(5), 646–674. https://doi.org/10.1016/j.cell.2011.02.013.
Bainbridge, P. (2013). Wound healing and the role of fibroblasts. Journal of Wound Care, 22(8), 407–408, 410-412. https://doi.org/10.12968/jowc.2013.22.8.407.
Kalluri. (2016). The biology and function of fibroblasts in cancer. Nature, 16(9), 582–598.
Unsworth, A., Anderson, R., & Britt, K. (2014). Stromal fibroblasts and the immune microenvironment: partners in mammary gland biology and pathology? Journal of Mammary Gland Biology and Neoplasia, 19(2), 169–182. https://doi.org/10.1007/s10911-014-9326-8.
Visvader, J. E., & Stingl, J. (2014). Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes & Development, 28(11), 1143–1158. https://doi.org/10.1101/gad.242511.114.
Polyak, K., & Kalluri, R. (2010). The role of the microenvironment in mammary gland development and cancer. Cold Spring Harbor Perspectives in Biology, 2(11), a003244. https://doi.org/10.1101/cshperspect.a003244.
Fleming, J. M., Long, E. L., Ginsburg, E., Gerscovich, D., Meltzer, P. S., & Vonderhaar, B. K. (2008). Interlobular and intralobular mammary stroma: genotype may not reflect phenotype. BMC Cell Biology, 9, 46. https://doi.org/10.1186/1471-2121-9-46.
Morsing, M., Klitgaard, M. C., Jafari, A., Villadsen, R., Kassem, M., Petersen, O. W., et al. (2016). Evidence of two distinct functionally specialized fibroblast lineages in breast stroma. Breast Cancer Research, 18(1), 108. https://doi.org/10.1186/s13058-016-0769-2.
Inman, J. L., Robertson, C., Mott, J. D., & Bissell, M. J. (2015). Mammary gland development: cell fate specification, stem cells and the microenvironment. Development, 142(6), 1028–1042. https://doi.org/10.1242/dev.087643.
Bussard, K. M., Mutkus, L., Stumpf, K., Gomez-Manzano, C., & Marini, F. C. (2016). Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Research, 18(1), 84. https://doi.org/10.1186/s13058-016-0740-2.
Osterreicher, C. H., Penz-Osterreicher, M., Grivennikov, S. I., Guma, M., Koltsova, E. K., Datz, C., et al. (2011). Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proceedings of the National Academy of Sciences of the United States of America, 108(1), 308–313. https://doi.org/10.1073/pnas.1017547108.
Lv, F. J., Tuan, R. S., Cheung, K. M., & Leung, V. Y. (2014). Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells, 32(6), 1408–1419. https://doi.org/10.1002/stem.1681.
Meng, M. B., Zaorsky, N. G., Deng, L., Wang, H. H., Chao, J., Zhao, L. J., et al. (2015). Pericytes: a double-edged sword in cancer therapy. Future Oncology, 11(1), 169–179. https://doi.org/10.2217/fon.14.123.
Su, S., Chen, J., Yao, H., Liu, J., Yu, S., Lao, L., et al. (2018). CD10(+)GPR77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell, 172(4), 841–856.e816. https://doi.org/10.1016/j.cell.2018.01.009.
Brechbuhl, H. M., Finlay-Schultz, J., Yamamoto, T. M., Gillen, A. E., Cittelly, D. M., Tan, A. C., et al. (2017). Fibroblast subtypes regulate responsiveness of luminal breast cancer to estrogen. Clinical Cancer Research, 23(7), 1710–1721. https://doi.org/10.1158/1078-0432.ccr-15-2851.
Tchou, J., Kossenkov, A. V., Chang, L., Satija, C., Herlyn, M., Showe, L. C., et al. (2012). Human breast cancer associated fibroblasts exhibit subtype specific gene expression profiles. BMC Medical Genomics, 5, 39–39. https://doi.org/10.1186/1755-8794-5-39.
Busch, S., Andersson, D., Bom, E., Walsh, C., Stahlberg, A., & Landberg, G. (2017). Cellular organization and molecular differentiation model of breast cancer-associated fibroblasts. Molecular Cancer, 16(1), 73. https://doi.org/10.1186/s12943-017-0642-7.
Jotzu, C., Alt, E., Welte, G., Li, J., Hennessy, B. T., Devarajan, E., et al. (2011). Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors. Cellular Oncology (Dordrecht), 34(1), 55–67. https://doi.org/10.1007/s13402-011-0012-1.
Cho, J. A., Park, H., Lim, E. H., & Lee, K. W. (2012). Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. International Journal of Oncology, 40(1), 130–138. https://doi.org/10.3892/ijo.2011.1193.
Weber, C. E., Kothari, A. N., Wai, P. Y., Li, N. Y., Driver, J., Zapf, M. A., et al. (2015). Osteopontin mediates an MZF1-TGF-beta1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene, 34(37), 4821–4833. https://doi.org/10.1038/onc.2014.410.
Avgustinova, A., Iravani, M., Robertson, D., Fearns, A., Gao, Q., Klingbeil, P., et al. (2016). Tumour cell-derived Wnt7a recruits and activates fibroblasts to promote tumour aggressiveness. Nature Communications, 7, 10305. https://doi.org/10.1038/ncomms10305.
Chen, J. Y., Li, C. F., Kuo, C. C., Tsai, K. K., Hou, M. F., & Hung, W. C. (2014). Cancer/stroma interplay via cyclooxygenase-2 and indoleamine 2,3-dioxygenase promotes breast cancer progression. Breast Cancer Research, 16(4), 410. https://doi.org/10.1186/s13058-014-0410-1.
Mishra, P. J., Mishra, P. J., Humeniuk, R., Medina, D. J., Alexe, G., Mesirov, J. P., et al. (2008). Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Research, 68(11), 4331–4339. https://doi.org/10.1158/0008-5472.can-08-0943.
Kidd, S., Spaeth, E., Watson, K., Burks, J., Lu, H., Klopp, A., et al. (2012). Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One, 7(2), e30563. https://doi.org/10.1371/journal.pone.0030563.
Dirat, B., Bochet, L., Dabek, M., Daviaud, D., Dauvillier, S., Majed, B., et al. (2011). Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Research, 71(7), 2455–2465. https://doi.org/10.1158/0008-5472.can-10-3323.
Bochet, L., Lehuede, C., Dauvillier, S., Wang, Y. Y., Dirat, B., Laurent, V., et al. (2013). Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Research, 73(18), 5657–5668. https://doi.org/10.1158/0008-5472.can-13-0530.
Kojima, Y., Acar, A., Eaton, E. N., Mellody, K. T., Scheel, C., Ben-Porath, I., et al. (2010). Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proceedings of the National Academy of Sciences of the United States of America, 107(46), 20009–20014. https://doi.org/10.1073/pnas.1013805107.
Nair, N., Calle, A. S., Zahra, M. H., Prieto-Vila, M., Oo, A. K. K., Hurley, L., et al. (2017). A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment. Scientific Reports, 7(1), 6838. https://doi.org/10.1038/s41598-017-07144-5.
LeBleu, V. S., Taduri, G., O'Connell, J., Teng, Y., Cooke, V. G., Woda, C., et al. (2013). Origin and function of myofibroblasts in kidney fibrosis. Nature Medicine, 19(8), 1047–1053. https://doi.org/10.1038/nm.3218.
Zarzynska, J. M. (2014). Two faces of TGF-beta1 in breast cancer. Mediators of Inflammation, 2014, 141747. https://doi.org/10.1155/2014/141747.
Kakarla, S., Song, X.-T., & Gottschalk, S. (2012). Cancer-associated fibroblasts as targets for immunotherapy. Immunotherapy, 4(11), 1129–1138. https://doi.org/10.2217/imt.12.112.
Shangguan, L., Ti, X., Krause, U., Hai, B., Zhao, Y., Yang, Z., et al. (2012). Inhibition of TGF-beta/Smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and abolishes their protumor effects. Stem Cells, 30(12), 2810–2819. https://doi.org/10.1002/stem.1251.
Gao, M. Q., Kim, B. G., Kang, S., Choi, Y. P., Yoon, J. H., & Cho, N. H. (2013). Human breast cancer-associated fibroblasts enhance cancer cell proliferation through increased TGF-alpha cleavage by ADAM17. Cancer Letters, 336(1), 240–246. https://doi.org/10.1016/j.canlet.2013.05.011.
Guido, C., Whitaker-Menezes, D., Capparelli, C., Balliet, R., Lin, Z., Pestell, R. G., et al. (2012). Metabolic reprogramming of cancer-associated fibroblasts by TGF-beta drives tumor growth: connecting TGF-beta signaling with "Warburg-like" cancer metabolism and L-lactate production. Cell Cycle, 11(16), 3019–3035. https://doi.org/10.4161/cc.21384.
Martinez-Outschoorn, U. E., Prisco, M., Ertel, A., Tsirigos, A., Lin, Z., Pavlides, S., et al. (2011). Ketones and lactate increase cancer cell "stemness," driving recurrence, metastasis and poor clinical outcome in breast cancer: achieving personalized medicine via Metabolo-Genomics. Cell Cycle, 10(8), 1271–1286. https://doi.org/10.4161/cc.10.8.15330.
Zhang, D., Wang, Y., Shi, Z., Liu, J., Sun, P., Hou, X., et al. (2015). Metabolic reprogramming of cancer-associated fibroblasts by IDH3alpha downregulation. Cell Reports, 10(8), 1335–1348. https://doi.org/10.1016/j.celrep.2015.02.006.
Yan, W., Wu, X., Zhou, W., Fong, M. Y., Cao, M., Liu, J., et al. (2018). Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nature Cell Biology, 20(5), 597–609. https://doi.org/10.1038/s41556-018-0083-6.
Donnarumma, E., Fiore, D., Nappa, M., Roscigno, G., Adamo, A., Iaboni, M., et al. (2017). Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget, 8(12), 19592–19608. https://doi.org/10.18632/oncotarget.14752.
Reya, T., Morrison, S. J., Clarke, M. F., & Weissman, I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature, 414(6859), 105–111. https://doi.org/10.1038/35102167.
Peiris-Pages, M., Sotgia, F., & Lisanti, M. P. (2015). Chemotherapy induces the cancer-associated fibroblast phenotype, activating paracrine Hedgehog-GLI signalling in breast cancer cells. Oncotarget, 6(13), 10728–10745. https://doi.org/10.18632/oncotarget.3828.
Zhao, X. L., Lin, Y., Jiang, J., Tang, Z., Yang, S., Lu, L., et al. (2017). High-mobility group box 1 released by autophagic cancer-associated fibroblasts maintains the stemness of luminal breast cancer cells. The Journal of Pathology, 243(3), 376–389. https://doi.org/10.1002/path.4958.
Tsuyada, A., Chow, A., Wu, J., Somlo, G., Chu, P., Loera, S., et al. (2012). CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Research, 72(11), 2768–2779. https://doi.org/10.1158/0008-5472.can-11-3567.
Cazet, A. S., Hui, M. N., Elsworth, B. L., Wu, S. Z., Roden, D., Chan, C. L., et al. (2018). Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nature Communications, 9(1), 2897. https://doi.org/10.1038/s41467-018-05220-6.
Boesch, M., Onder, L., Cheng, H.-W., Novkovic, M., Mörbe, U., Sopper, S., et al. (2018). Interleukin 7-expressing fibroblasts promote breast cancer growth through sustenance of tumor cell stemness. OncoImmunology, 7(4), e1414129. https://doi.org/10.1080/2162402X.2017.1414129.
Sansone, P., Savini, C., Kurelac, I., Chang, Q., Amato, L. B., Strillacci, A., et al. (2017). Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proceedings of the National Academy of Sciences of the United States of America, 114(43), E9066–e9075. https://doi.org/10.1073/pnas.1704862114.
De Wever, O., Van Bockstal, M., Mareel, M., Hendrix, A., & Bracke, M. (2014). Carcinoma-associated fibroblasts provide operational flexibility in metastasis. Seminars in Cancer Biology, 25, 33–46. https://doi.org/10.1016/j.semcancer.2013.12.009.
Dittmer, A., & Dittmer, J. (2018). Long-term exposure to carcinoma-associated fibroblasts makes breast cancer cells addictive to integrin beta1. Oncotarget, 9(31), 22079–22094. https://doi.org/10.18632/oncotarget.25183.
Orimo, A., Gupta, P. B., Sgroi, D. C., Arenzana-Seisdedos, F., Delaunay, T., Naeem, R., et al. (2005). Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell, 121(3), 335–348. https://doi.org/10.1016/j.cell.2005.02.034.
Al-Rakan, M. A., Colak, D., Hendrayani, S. F., Al-Bakheet, A., Al-Mohanna, F. H., Kaya, N., et al. (2013). Breast stromal fibroblasts from histologically normal surgical margins are pro-carcinogenic. The Journal of Pathology, 231(4), 457–465. https://doi.org/10.1002/path.4256.
Chen, L. C., Tu, S. H., Huang, C. S., Chen, C. S., Ho, C. T., Lin, H. W., et al. (2012). Human breast cancer cell metastasis is attenuated by lysyl oxidase inhibitors through down-regulation of focal adhesion kinase and the paxillin-signaling pathway. Breast Cancer Research and Treatment, 134(3), 989–1004. https://doi.org/10.1007/s10549-012-1986-8.
Tyan, S. W., Hsu, C. H., Peng, K. L., Chen, C. C., Kuo, W. H., Lee, E. Y., et al. (2012). Breast cancer cells induce stromal fibroblasts to secrete ADAMTS1 for cancer invasion through an epigenetic change. PLoS One, 7(4), e35128. https://doi.org/10.1371/journal.pone.0035128.
Pinto, M. P., Dye, W. W., Jacobsen, B. M., & Horwitz, K. B. (2014). Malignant stroma increases luminal breast cancer cell proliferation and angiogenesis through platelet-derived growth factor signaling. BMC Cancer, 14, 735. https://doi.org/10.1186/1471-2407-14-735.
Adams, E. F., Newton, C. J., Braunsberg, H., Shaikh, N., Ghilchik, M., & James, V. H. (1988). Effects of human breast fibroblasts on growth and 17 beta-estradiol dehydrogenase activity of MCF-7 cells in culture. Breast Cancer Research and Treatment, 11(2), 165–172.
Cheng, G., Fan, X., Hao, M., Wang, J., Zhou, X., & Sun, X. (2016). Higher levels of TIMP-1 expression are associated with a poor prognosis in triple-negative breast cancer. Molecular Cancer, 15(1), 30. https://doi.org/10.1186/s12943-016-0515-5.
Rasmussen, A. A., & Cullen, K. J. (1998). Paracrine/autocrine regulation of breast cancer by the insulin-like growth factors. Breast Cancer Research and Treatment, 47(3), 219–233.
Bernard, S., Myers, M., Fang, W. B., Zinda, B., Smart, C., Lambert, D., et al. (2018). CXCL1 derived from mammary fibroblasts promotes progression of mammary lesions to invasive carcinoma through CXCR2 dependent mechanisms. Journal of Mammary Gland Biology and Neoplasia. https://doi.org/10.1007/s10911-018-9407-1.
Jin, K., Pandey, N. B., & Popel, A. S. (2017). Crosstalk between stromal components and tumor cells of TNBC via secreted factors enhances tumor growth and metastasis. Oncotarget, 8(36), 60210–60222. https://doi.org/10.18632/oncotarget.19417.
Pickup, M. W., Mouw, J. K., & Weaver, V. M. (2014). The extracellular matrix modulates the hallmarks of cancer. EMBO Reports, 15(12), 1243–1253. https://doi.org/10.15252/embr.201439246.
Bergamaschi, A., Tagliabue, E., Sørlie, T., Naume, B., Triulzi, T., Orlandi, R., et al. (2008). Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. The Journal of Pathology, 214(3), 357–367. https://doi.org/10.1002/path.2278.
Robertson, C. (2016). The extracellular matrix in breast cancer predicts prognosis through composition, splicing, and crosslinking. Experimental Cell Research, 343(1), 73–81. https://doi.org/10.1016/j.yexcr.2015.11.009.
Boraschi-Diaz, I., Wang, J., Mort, J. S., & Komarova, S. V. (2017). Collagen type I as a ligand for receptor-mediated signaling. [Review]. Frontiers in Physics, 5(12). https://doi.org/10.3389/fphy.2017.00012.
Heino, J. (2014). Cellular signaling by collagen-binding integrins. Advances in Experimental Medicine and Biology, 819, 143–155. https://doi.org/10.1007/978-94-017-9153-3_10.
Bhogal, R. K., Stoica, C. M., McGaha, T. L., & Bona, C. A. (2005). Molecular aspects of regulation of collagen gene expression in fibrosis. Journal of Clinical Immunology, 25(6), 592–603. https://doi.org/10.1007/s10875-005-7827-3.
Bates, A. L., Pickup, M. W., Hallett, M. A., Dozier, E. A., Thomas, S., & Fingleton, B. (2015). Stromal matrix metalloproteinase 2 regulates collagen expression and promotes the outgrowth of experimental metastases. The Journal of Pathology, 235(5), 773–783. https://doi.org/10.1002/path.4493.
Kim, S. H., Lee, H. Y., Jung, S. P., Kim, S., Lee, J. E., Nam, S. J., et al. (2014). Role of secreted type I collagen derived from stromal cells in two breast cancer cell lines. Oncology Letters, 8(2), 507–512. https://doi.org/10.3892/ol.2014.2199.
Liu, J., Shen, J. X., Wu, H. T., Li, X. L., Wen, X. F., Du, C. W., et al. (2018). Collagen 1A1 (COL1A1) promotes metastasis of breast cancer and is a potential therapeutic target. Discovery Medicine, 25(139), 211–223.
Krishnamachary, B., Stasinopoulos, I., Kakkad, S., Penet, M. F., Jacob, D., Wildes, F., et al. (2017). Breast cancer cell cyclooxygenase-2 expression alters extracellular matrix structure and function and numbers of cancer associated fibroblasts. Oncotarget, 8(11), 17981–17994. https://doi.org/10.18632/oncotarget.14912.
Badaoui, M., Mimsy-Julienne, C., Saby, C., Van Gulick, L., Peretti, M., Jeannesson, P., et al. (2018). Collagen type 1 promotes survival of human breast cancer cells by overexpressing Kv10.1 potassium and Orai1 calcium channels through DDR1-dependent pathway. Oncotarget, 9(37), 24653–24671. https://doi.org/10.18632/oncotarget.19065.
Barcus, C. E., O'Leary, K. A., Brockman, J. L., Rugowski, D. E., Liu, Y., Garcia, N., et al. (2017). Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells. Breast Cancer Research, 19(1), 9. https://doi.org/10.1186/s13058-017-0801-1.
Conklin, M. W., Eickhoff, J. C., Riching, K. M., Pehlke, C. A., Eliceiri, K. W., Provenzano, P. P., et al. (2011). Aligned collagen is a prognostic signature for survival in human breast carcinoma. The American Journal of Pathology, 178(3), 1221–1232. https://doi.org/10.1016/j.ajpath.2010.11.076.
Morris, B. A., Burkel, B., Ponik, S. M., Fan, J., Condeelis, J. S., Aguirre-Ghiso, J. A., et al. (2016). Collagen matrix density drives the metabolic shift in breast cancer cells. EBioMedicine, 13, 146–156. https://doi.org/10.1016/j.ebiom.2016.10.012.
Xiong, G., Deng, L., Zhu, J., Rychahou, P. G., & Xu, R. (2014). Prolyl-4-hydroxylase alpha subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer, 14, 1. https://doi.org/10.1186/1471-2407-14-1.
Karousou, E., D'Angelo, M. L., Kouvidi, K., Vigetti, D., Viola, M., Nikitovic, D., et al. (2014). Collagen VI and hyaluronan: the common role in breast cancer. BioMed Research International, 2014, 606458. https://doi.org/10.1155/2014/606458.
Castro-Sanchez, L., Soto-Guzman, A., Navarro-Tito, N., Martinez-Orozco, R., & Salazar, E. P. (2010). Native type IV collagen induces cell migration through a CD9 and DDR1-dependent pathway in MDA-MB-231 breast cancer cells. European Journal of Cell Biology, 89(11), 843–852. https://doi.org/10.1016/j.ejcb.2010.07.004.
Mazouni, C., Arun, B., Andre, F., Ayers, M., Krishnamurthy, S., Wang, B., et al. (2008). Collagen IV levels are elevated in the serum of patients with primary breast cancer compared to healthy volunteers. British Journal of Cancer, 99(1), 68–71. https://doi.org/10.1038/sj.bjc.6604443.
Brodsky, A. S., Xiong, J., Yang, D., Schorl, C., Fenton, M. A., Graves, T. A., et al. (2016). Identification of stromal ColXalpha1 and tumor-infiltrating lymphocytes as putative predictive markers of neoadjuvant therapy in estrogen receptor-positive/HER2-positive breast cancer. BMC Cancer, 16, 274. https://doi.org/10.1186/s12885-016-2302-5.
Wang, J. P., & Hielscher, A. (2017). Fibronectin: how its aberrant expression in tumors may improve therapeutic targeting. Journal of Cancer, 8(4), 674–682. https://doi.org/10.7150/jca.16901.
Insua-Rodriguez, J., & Oskarsson, T. (2016). The extracellular matrix in breast cancer. Advanced Drug Delivery Reviews, 97, 41–55. https://doi.org/10.1016/j.addr.2015.12.017.
Multhaupt, H. A., Leitinger, B., Gullberg, D., & Couchman, J. R. (2016). Extracellular matrix component signaling in cancer. Advanced Drug Delivery Reviews, 97, 28–40. https://doi.org/10.1016/j.addr.2015.10.013.
Rybak, J. N., Roesli, C., Kaspar, M., Villa, A., & Neri, D. (2007). The extra-domain A of fibronectin is a vascular marker of solid tumors and metastases. Cancer Research, 67(22), 10948–10957. https://doi.org/10.1158/0008-5472.can-07-1436.
Ignotz, R. A., & Massague, J. (1986). Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. The Journal of Biological Chemistry, 261(9), 4337–4345.
Mulsow, J. J., Watson, R. W., Fitzpatrick, J. M., & O'Connell, P. R. (2005). Transforming growth factor-beta promotes pro-fibrotic behavior by serosal fibroblasts via PKC and ERK1/2 mitogen activated protein kinase cell signaling. Annals of Surgery, 242(6), 880–887 discussion 887-889.
Czaja, M. J., Weiner, F. R., Eghbali, M., Giambrone, M. A., Eghbali, M., & Zern, M. A. (1987). Differential effects of gamma-interferon on collagen and fibronectin gene expression. The Journal of Biological Chemistry, 262(27), 13348–13351.
Erdogan, B., Ao, M., White, L. M., Means, A. L., Brewer, B. M., Yang, L., et al. (2017). Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. The Journal of Cell Biology, 216(11), 3799–3816. https://doi.org/10.1083/jcb.201704053.
Yao, E. S., Zhang, H., Chen, Y. Y., Lee, B., Chew, K., Moore, D., et al. (2007). Increased beta1 integrin is associated with decreased survival in invasive breast cancer. Cancer Research, 67(2), 659–664. https://doi.org/10.1158/0008-5472.can-06-2768.
Li, C. L., Yang, D., Cao, X., Wang, F., Hong, D. Y., Wang, J., et al. (2017). Fibronectin induces epithelial-mesenchymal transition in human breast cancer MCF-7 cells via activation of calpain. Oncology Letters, 13(5), 3889–3895. https://doi.org/10.3892/ol.2017.5896.
Balanis, N., Wendt, M. K., Schiemann, B. J., Wang, Z., Schiemann, W. P., & Carlin, C. R. (2013). Epithelial to mesenchymal transition promotes breast cancer progression via a fibronectin-dependent STAT3 signaling pathway. The Journal of Biological Chemistry, 288(25), 17954–17967. https://doi.org/10.1074/jbc.M113.475277.
Hong, H., Zhou, T., Fang, S., Jia, M., Xu, Z., Dai, Z., et al. (2014). Pigment epithelium-derived factor (PEDF) inhibits breast cancer metastasis by down-regulating fibronectin. Breast Cancer Research and Treatment, 148(1), 61–72. https://doi.org/10.1007/s10549-014-3154-9.
He, Z. H., Lei, Z., Zhen, Y., Gong, W., Huang, B., Yuan, Y., et al. (2014). Adeno-associated virus-mediated expression of recombinant CBD-HepII polypeptide of human fibronectin inhibits metastasis of breast cancer. Breast Cancer Research and Treatment, 143(1), 33–45. https://doi.org/10.1007/s10549-013-2783-8.
Park, C. C., Zhang, H., Pallavicini, M., Gray, J. W., Baehner, F., Park, C. J., et al. (2006). Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Research, 66(3), 1526–1535. https://doi.org/10.1158/0008-5472.can-05-3071.
Sampayo, R. G., Toscani, A. M., Rubashkin, M. G., Thi, K., Masullo, L. A., Violi, I. L., et al. (2018). Fibronectin rescues estrogen receptor alpha from lysosomal degradation in breast cancer cells. The Journal of Cell Biology, 217(8), 2777–2798. https://doi.org/10.1083/jcb.201703037.
Tucker, R. P., & Chiquet-Ehrismann, R. (2009). The regulation of tenascin expression by tissue microenvironments. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1793(5), 888–892. https://doi.org/10.1016/j.bbamcr.2008.12.012.
Hancox, R. A., Allen, M. D., Holliday, D. L., Edwards, D. R., Pennington, C. J., Guttery, D. S., et al. (2009). Tumour-associated tenascin-C isoforms promote breast cancer cell invasion and growth by matrix metalloproteinase-dependent and independent mechanisms. Breast Cancer Research, 11(2), R24. https://doi.org/10.1186/bcr2251.
Yang, Z., Ni, W., Cui, C., Fang, L., & Xuan, Y. (2017). Tenascin C is a prognostic determinant and potential cancer-associated fibroblasts marker for breast ductal carcinoma. Experimental and Molecular Pathology, 102(2), 262–267. https://doi.org/10.1016/j.yexmp.2017.02.012.
Adams, M., Jones, J. L., Walker, R. A., Pringle, J. H., & Bell, S. C. (2002). Changes in tenascin-C isoform expression in invasive and preinvasive breast disease. Cancer Research, 62(11), 3289–3297.
Oskarsson, T., Acharyya, S., Zhang, X. H. F., Vanharanta, S., Tavazoie, S. F., Morris, P. G., et al. (2011). Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. [Article]. Nature Medicine, 17, 867. https://doi.org/10.1038/nm.2379.
Degen, M., Brellier, F., Schenk, S., Driscoll, R., Zaman, K., Stupp, R., et al. (2008). Tenascin-W, a new marker of cancer stroma, is elevated in sera of colon and breast cancer patients. International Journal of Cancer, 122(11), 2454–2461. https://doi.org/10.1002/ijc.23417.
Degen, M., Brellier, F., Kain, R., Ruiz, C., Terracciano, L., Orend, G., et al. (2007). Tenascin-W is a novel marker for activated tumor stroma in low-grade human breast cancer and influences cell behavior. Cancer Research, 67(19), 9169–9179. https://doi.org/10.1158/0008-5472.can-07-0666.
Brellier, F., Martina, E., Degen, M., Heuze-Vourc'h, N., Petit, A., Kryza, T., et al. (2012). Tenascin-W is a better cancer biomarker than tenascin-C for most human solid tumors. BMC Clinical Pathology, 12, 14. https://doi.org/10.1186/1472-6890-12-14.
Chiovaro, F., Martina, E., Bottos, A., Scherberich, A., Hynes, N. E., & Chiquet-Ehrismann, R. (2015). Transcriptional regulation of tenascin-W by TGF-beta signaling in the bone metastatic niche of breast cancer cells. International Journal of Cancer, 137(8), 1842–1854. https://doi.org/10.1002/ijc.29565.
Baker, A. M., Bird, D., Lang, G., Cox, T. R., & Erler, J. T. (2013). Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene, 32(14), 1863–1868. https://doi.org/10.1038/onc.2012.202.
Provenzano, P. P., Cuevas, C., Chang, A. E., Goel, V. K., Von Hoff, D. D., & Hingorani, S. R. (2012). Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell, 21(3), 418–429. https://doi.org/10.1016/j.ccr.2012.01.007.
Provenzano, P. P., Eliceiri, K. W., Campbell, J. M., Inman, D. R., White, J. G., & Keely, P. J. (2006). Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Medicine, 4(1), 38. https://doi.org/10.1186/1741-7015-4-38.
Levental, K. R., Yu, H., Kass, L., Lakins, J. N., Egeblad, M., Erler, J. T., et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell, 139(5), 891–906. https://doi.org/10.1016/j.cell.2009.10.027.
Wells, R. G. (2008). The role of matrix stiffness in regulating cell behavior. Hepatology, 47(4), 1394–1400. https://doi.org/10.1002/hep.22193.
Mouw, J. K., Yui, Y., Damiano, L., Bainer, R. O., Lakins, J. N., Acerbi, I., et al. (2014). Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nature Medicine, 20(4), 360–367. https://doi.org/10.1038/nm.3497.
Pickup, M. W., Laklai, H., Acerbi, I., Owens, P., Gorska, A. E., Chytil, A., et al. (2013). Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-beta-deficient mouse mammary carcinomas. Cancer Research, 73(17), 5336–5346. https://doi.org/10.1158/0008-5472.can-13-0012.
Erler, J. T., Bennewith, K. L., Nicolau, M., Dornhofer, N., Kong, C., Le, Q. T., et al. (2006). Lysyl oxidase is essential for hypoxia-induced metastasis. Nature, 440(7088), 1222–1226. https://doi.org/10.1038/nature04695.
Tang, X., Hou, Y., Yang, G., Wang, X., Tang, S., Du, Y. E., et al. (2016). Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling. Cell Death and Differentiation, 23(1), 132–145. https://doi.org/10.1038/cdd.2015.78.
El-Mohri, H., Wu, Y., Mohanty, S., & Ghosh, G. (2017). Impact of matrix stiffness on fibroblast function. Materials Science & Engineering. C, Materials for Biological Applications, 74, 146–151. https://doi.org/10.1016/j.msec.2017.02.001.
Asano, S., Ito, S., Takahashi, K., Furuya, K., Kondo, M., Sokabe, M., et al. (2017). Matrix stiffness regulates migration of human lung fibroblasts. Physiological Reports, 5(9). https://doi.org/10.14814/phy2.13281.
Basset, P., Bellocq, J. P., Wolf, C., Stoll, I., Hutin, P., Limacher, J. M., et al. (1990). A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature, 348(6303), 699–704. https://doi.org/10.1038/348699a0.
Têtu, B., Brisson, J., Wang, C. S., Lapointe, H., Beaudry, G., Blanchette, C., et al. (2006). The influence of MMP-14, TIMP-2 and MMP-2 expression on breast cancer prognosis. [journal article]. Breast Cancer Research, 8(3), R28. https://doi.org/10.1186/bcr1503.
Radisky, E. S., & Radisky, D. C. (2015). Matrix metalloproteinases as breast cancer drivers and therapeutic targets. Frontiers in Bioscience (Landmark edition), 20, 1144–1163.
Stuelten, C. H., DaCosta Byfield, S., Arany, P. R., Karpova, T. S., Stetler-Stevenson, W. G., & Roberts, A. B. (2005). Breast cancer cells induce stromal fibroblasts to express MMP-9 via secretion of TNF-alpha and TGF-beta. Journal of Cell Science, 118(Pt 10), 2143–2153. https://doi.org/10.1242/jcs.02334.
Saad, S., Gottlieb, D. J., Bradstock, K. F., Overall, C. M., & Bendall, L. J. (2002). Cancer cell-associated fibronectin induces release of matrix metalloproteinase-2 from normal fibroblasts. Cancer Research, 62, 283–289.
Lochter, A., Galosy, S., Muschler, J., Freedman, N., Werb, Z., & Bissell, M. J. (1997). Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. The Journal of Cell Biology, 139(7), 1861–1872.
Xu, H., Li, M., Zhou, Y., Wang, F., Li, X., Wang, L., et al. (2016). S100A4 participates in epithelial-mesenchymal transition in breast cancer via targeting MMP2. Tumour Biology, 37(3), 2925–2932. https://doi.org/10.1007/s13277-015-3709-3.
Liss, M., Sreedhar, N., Keshgegian, A., Sauter, G., Chernick, M. R., Prendergast, G. C., et al. (2009). Tissue inhibitor of metalloproteinase-4 is elevated in early-stage breast cancers with accelerated progression and poor clinical course. The American Journal of Pathology, 175(3), 940–946. https://doi.org/10.2353/ajpath.2009.081094.
Gong, Y., Scott, E., Lu, R., Xu, Y., Oh, W. K., & Yu, Q. (2013). TIMP-1 promotes accumulation of cancer associated fibroblasts and cancer progression. PLoS One, 8(10), e77366. https://doi.org/10.1371/journal.pone.0077366.
Song, T., Dou, C., Jia, Y., Tu, K., & Zheng, X. (2015). TIMP-1 activated carcinoma-associated fibroblasts inhibit tumor apoptosis by activating SDF1/CXCR4 signaling in hepatocellular carcinoma. Oncotarget, 6(14), 12061–12079. https://doi.org/10.18632/oncotarget.3616.
Dang, T. T., Prechtl, A. M., & Pearson, G. W. (2011). Breast cancer subtype-specific interactions with the microenvironment dictate mechanisms of invasion. Cancer Research, 71(21), 6857–6866. https://doi.org/10.1158/0008-5472.can-11-1818.
Hu, M., Yao, J., Carroll, D. K., Weremowicz, S., Chen, H., Carrasco, D., et al. (2008). Regulation of in situ to invasive breast carcinoma transition. Cancer Cell, 13(5), 394–406. https://doi.org/10.1016/j.ccr.2008.03.007.
Osuala, K. O., Sameni, M., Shah, S., Aggarwal, N., Simonait, M. L., Franco, O. E., et al. (2015). Il-6 signaling between ductal carcinoma in situ cells and carcinoma-associated fibroblasts mediates tumor cell growth and migration. BMC Cancer, 15, 584. https://doi.org/10.1186/s12885-015-1576-3.
Yu, Y., Xiao, C. H., Tan, L. D., Wang, Q. S., Li, X. Q., & Feng, Y. M. (2014). Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-beta signalling. British Journal of Cancer, 110(3), 724–732. https://doi.org/10.1038/bjc.2013.768.
Takai, K., Le, A., Weaver, V. M., & Werb, Z. (2016). Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget, 7(50), 82889–82901. https://doi.org/10.18632/oncotarget.12658.
Bellomo, C., Caja, L., & Moustakas, A. (2016). Transforming growth factor β as regulator of cancer stemness and metastasis. British Journal of Cancer, 115(7), 761–769. https://doi.org/10.1038/bjc.2016.255.
Dvorak, K. M., Pettee, K. M., Rubinic-Minotti, K., Su, R., Nestor-Kalinoski, A., & Eisenmann, K. M. (2018). Carcinoma associated fibroblasts (CAFs) promote breast cancer motility by suppressing mammalian Diaphanous-related formin-2 (mDia2). PLoS One, 13(3), e0195278. https://doi.org/10.1371/journal.pone.0195278.
Ahirwar, D. K., Nasser, M. W., Ouseph, M. M., Elbaz, M., Cuitino, M. C., Kladney, R. D., et al. (2018). Fibroblast-derived CXCL12 promotes breast cancer metastasis by facilitating tumor cell intravasation. Oncogene. https://doi.org/10.1038/s41388-018-0263-7.
O'Connell, J. T., Sugimoto, H., Cooke, V. G., MacDonald, B. A., Mehta, A. I., LeBleu, V. S., et al. (2011). VEGF-A and tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proceedings of the National Academy of Sciences of the United States of America, 108(38), 16002–16007. https://doi.org/10.1073/pnas.1109493108.
Studebaker, A. W., Storci, G., Werbeck, J. L., Sansone, P., Sasser, A. K., Tavolari, S., et al. (2008). Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Research, 68(21), 9087–9095. https://doi.org/10.1158/0008-5472.can-08-0400.
Xu, K., Tian, X., Oh, S. Y., Movassaghi, M., Naber, S. P., Kuperwasser, C., et al. (2016). The fibroblast Tiam1-osteopontin pathway modulates breast cancer invasion and metastasis. Breast Cancer Research, 18(1), 14. https://doi.org/10.1186/s13058-016-0674-8.
Lowry, M. C., Gallagher, W. M., & O'Driscoll, L. (2015). The role of exosomes in breast cancer. Clinical Chemistry, 61(12), 1457–1465. https://doi.org/10.1373/clinchem.2015.240028.
Chen, Y., Zeng, C., Zhan, Y., Wang, H., Jiang, X., & Li, W. (2017). Aberrant low expression of p85α in stromal fibroblasts promotes breast cancer cell metastasis through exosome-mediated paracrine Wnt10b. [original article]. Oncogene, 36, 4692. https://doi.org/10.1038/onc.2017.100.
Luga, V., Zhang, L., Viloria-Petit, A. M., Ogunjimi, A. A., Inanlou, M. R., Chiu, E., et al. (2012). Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell, 151(7), 1542–1556. https://doi.org/10.1016/j.cell.2012.11.024.
Shimoda, M., Principe, S., Jackson, H. W., Luga, V., Fang, H., Molyneux, S. D., et al. (2014). Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state. Nature Cell Biology, 16(9), 889–901. https://doi.org/10.1038/ncb3021.
Nabet, B. Y., Qiu, Y., Shabason, J. E., Wu, T. J., Yoon, T., Kim, B. C., et al. (2017). Exosome RNA unshielding couples stromal activation to pattern recognition receptor signaling in cancer. Cell, 170(2), 352–366.e313. https://doi.org/10.1016/j.cell.2017.06.031.
Choi, Y. P., Lee, J. H., Gao, M. Q., Kim, B. G., Kang, S., Kim, S. H., et al. (2014). Cancer-associated fibroblast promote transmigration through endothelial brain cells in three-dimensional in vitro models. International Journal of Cancer, 135(9), 2024–2033. https://doi.org/10.1002/ijc.28848.
Gaggioli, C., Hooper, S., Hidalgo-Carcedo, C., Grosse, R., Marshall, J. F., Harrington, K., et al. (2007). Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nature Cell Biology, 9(12), 1392–1400. https://doi.org/10.1038/ncb1658.
Yang, N., Mosher, R., Seo, S., Beebe, D., & Friedl, A. (2011). Syndecan-1 in breast cancer stroma fibroblasts regulates extracellular matrix fiber organization and carcinoma cell motility. The American Journal of Pathology, 178(1), 325–335. https://doi.org/10.1016/j.ajpath.2010.11.039.
Chute, C., Yang, X., Meyer, K., Yang, N., O'Neil, K., Kasza, I., et al. (2018). Syndecan-1 induction in lung microenvironment supports the establishment of breast tumor metastases. Breast Cancer Research, 20(1), 66. https://doi.org/10.1186/s13058-018-0995-x.
Corsa, C. A., Brenot, A., Grither, W. R., Van Hove, S., Loza, A. J., Zhang, K., et al. (2016). The action of Discoidin domain receptor 2 in basal tumor cells and stromal cancer-associated fibroblasts is critical for breast cancer metastasis. Cell Reports, 15(11), 2510–2523. https://doi.org/10.1016/j.celrep.2016.05.033.
Farmaki, E., Chatzistamou, I., Kaza, V., & Kiaris, H. (2016). A CCL8 gradient drives breast cancer cell dissemination. Oncogene, 35(49), 6309–6318. https://doi.org/10.1038/onc.2016.161.
Wang, K., Wu, F., Seo, B. R., Fischbach, C., Chen, W., Hsu, L., et al. (2017). Breast cancer cells alter the dynamics of stromal fibronectin-collagen interactions. Matrix Biology, 60-61, 86–95. https://doi.org/10.1016/j.matbio.2016.08.001.
Dvorak, H. F. (1986). Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. The New England Journal of Medicine, 315(26), 1650–1659. https://doi.org/10.1056/nejm198612253152606.
Hanahan, D., & Coussens, L. M. (2012). Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell, 21(3), 309–322. https://doi.org/10.1016/j.ccr.2012.02.022.
Neuzillet, C., Tijeras-Raballand, A., Cohen, R., Cros, J., Faivre, S., Raymond, E., et al. (2015). Targeting the TGFbeta pathway for cancer therapy. Pharmacology & Therapeutics, 147, 22–31. https://doi.org/10.1016/j.pharmthera.2014.11.001.
Ziani, L., Chouaib, S., & Thiery, J. (2018). Alteration of the antitumor immune response by cancer-associated fibroblasts. Frontiers in Immunology, 9, 414. https://doi.org/10.3389/fimmu.2018.00414.
Kinoshita, T., Ishii, G., Hiraoka, N., Hirayama, S., Yamauchi, C., Aokage, K., et al. (2013). Forkhead box P3 regulatory T cells coexisting with cancer associated fibroblasts are correlated with a poor outcome in lung adenocarcinoma. Cancer Science, 104(4), 409–415. https://doi.org/10.1111/cas.12099.
Li, T., Yi, S., Liu, W., Jia, C., Wang, G., Hua, X., et al. (2013). Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Medical Oncology, 30(3), 663. https://doi.org/10.1007/s12032-013-0663-z.
Shen, C. C., Kang, Y. H., Zhao, M., He, Y., Cui, D. D., Fu, Y. Y., et al. (2014). WNT16B from ovarian fibroblasts induces differentiation of regulatory T cells through beta-catenin signal in dendritic cells. International Journal of Molecular Sciences, 15(7), 12928–12939. https://doi.org/10.3390/ijms150712928.
Takahashi, H., Sakakura, K., Kudo, T., Toyoda, M., Kaira, K., Oyama, T., et al. (2017). Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget, 8(5), 8633–8647. https://doi.org/10.18632/oncotarget.14374.
Fu, Z., Zuo, Y., Li, D., Xu, W., Li, D., Chen, H., et al. (2013). The crosstalk: tumor-infiltrating lymphocytes rich in regulatory T cells suppressed cancer-associated fibroblasts. Acta Oncologica, 52(8), 1760–1770. https://doi.org/10.3109/0284186X.2012.760847.
Allaoui, R., Bergenfelz, C., Mohlin, S., Hagerling, C., Salari, K., Werb, Z., et al. (2016). Cancer-associated fibroblast-secreted CXCL16 attracts monocytes to promote stroma activation in triple-negative breast cancers. Nature Communications, 7, 13050. https://doi.org/10.1038/ncomms13050.
Silzle, T., Kreutz, M., Dobler, M. A., Brockhoff, G., Knuechel, R., & Kunz-Schughart, L. A. (2003). Tumor-associated fibroblasts recruit blood monocytes into tumor tissue. European Journal of Immunology, 33(5), 1311–1320. https://doi.org/10.1002/eji.200323057.
Qian, B. Z., Li, J., Zhang, H., Kitamura, T., Zhang, J., Campion, L. R., et al. (2011). CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature, 475(7355), 222–225. https://doi.org/10.1038/nature10138.
Liao, D., Luo, Y., Markowitz, D., Xiang, R., & Reisfeld, R. A. (2009). Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One, 4(11), e7965. https://doi.org/10.1371/journal.pone.0007965.
Li, A., Chen, P., Leng, Y., & Kang, J. (2018). Histone deacetylase 6 regulates the immunosuppressive properties of cancer-associated fibroblasts in breast cancer through the STAT3-COX2-dependent pathway. Oncogene. https://doi.org/10.1038/s41388-018-0379-9.
Cohen, N., Shani, O., Raz, Y., Sharon, Y., Hoffman, D., Abramovitz, L., et al. (2017). Fibroblasts drive an immunosuppressive and growth-promoting microenvironment in breast cancer via secretion of Chitinase 3-like 1. Oncogene, 36(31), 4457–4468. https://doi.org/10.1038/onc.2017.65.
Costa, A., Kieffer, Y., Scholer-Dahirel, A., Pelon, F., Bourachot, B., Cardon, M., et al. (2018). Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell, 33(3), 463–479.e410. https://doi.org/10.1016/j.ccell.2018.01.011.
Panagopoulos, V., Leach, D. A., Zinonos, I., Ponomarev, V., Licari, G., Liapis, V., et al. (2017). Inflammatory peroxidases promote breast cancer progression in mice via regulation of the tumour microenvironment. International Journal of Oncology, 50(4), 1191–1200. https://doi.org/10.3892/ijo.2017.3883.
Lu, P., Weaver, V. M., & Werb, Z. (2012). The extracellular matrix: a dynamic niche in cancer progression. The Journal of Cell Biology, 196(4), 395–406. https://doi.org/10.1083/jcb.201102147.
Bae, Y. K., Kim, A., Kim, M. K., Choi, J. E., Kang, S. H., & Lee, S. J. (2013). Fibronectin expression in carcinoma cells correlates with tumor aggressiveness and poor clinical outcome in patients with invasive breast cancer. Human Pathology, 44(10), 2028–2037. https://doi.org/10.1016/j.humpath.2013.03.006.
Fernandez-Garcia, B., Eiro, N., Marin, L., Gonzalez-Reyes, S., Gonzalez, L. O., Lamelas, M. L., et al. (2014). Expression and prognostic significance of fibronectin and matrix metalloproteases in breast cancer metastasis. Histopathology, 64(4), 512–522. https://doi.org/10.1111/his.12300.
Acerbi, I., Cassereau, L., Dean, I., Shi, Q., Au, A., Park, C., et al. (2015). Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integrative Biology: Quantitative Biosciences from Nano to Macro, 7(10), 1120–1134. https://doi.org/10.1039/c5ib00040h.
Jachetti, E., Caputo, S., Mazzoleni, S., Brambillasca, C. S., Parigi, S. M., Grioni, M., et al. (2015). Tenascin-C protects cancer stem-like cells from immune surveillance by arresting T-cell activation. Cancer Research, 75(10), 2095–2108. https://doi.org/10.1158/0008-5472.can-14-2346.
Huang, J. Y., Cheng, Y. J., Lin, Y. P., Lin, H. C., Su, C. C., Juliano, R., et al. (2010). Extracellular matrix of glioblastoma inhibits polarization and transmigration of T cells: the role of tenascin-C in immune suppression. Journal of Immunology, 185(3), 1450–1459. https://doi.org/10.4049/jimmunol.0901352.
Tsunoda, T., Inada, H., Kalembeyi, I., Imanaka-Yoshida, K., Sakakibara, M., Okada, R., et al. (2003). Involvement of large tenascin-C splice variants in breast cancer progression. The American Journal of Pathology, 162(6), 1857–1867. https://doi.org/10.1016/s0002-9440(10)64320-9.
Kelsh, R., You, R., Horzempa, C., Zheng, M., & McKeown-Longo, P. J. (2014). Regulation of the innate immune response by fibronectin: synergism between the III-1 and EDA domains. PLoS One, 9(7), e102974. https://doi.org/10.1371/journal.pone.0102974.
Rossnagl, S., Altrock, E., Sens, C., Kraft, S., Rau, K., Milsom, M. D., et al. (2016). EDA-fibronectin originating from osteoblasts inhibits the immune response against cancer. PLoS Biology, 14(9), e1002562. https://doi.org/10.1371/journal.pbio.1002562.
Farmer, P., Bonnefoi, H., Anderle, P., Cameron, D., Wirapati, P., Becette, V., et al. (2009). A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nature Medicine, 15(1), 68–74. https://doi.org/10.1038/nm.1908.
Jia, D., Liu, Z., Deng, N., Tan, T. Z., Huang, R. Y., Taylor-Harding, B., et al. (2016). A COL11A1-correlated pan-cancer gene signature of activated fibroblasts for the prioritization of therapeutic targets. Cancer Letters, 382(2), 203–214. https://doi.org/10.1016/j.canlet.2016.09.001.
Cukierman, E., & Bassi, D. E. (2012). The mesenchymal tumor microenvironment. Cell Adhesion & Migration, 6(3), 285–296. https://doi.org/10.4161/cam.20210.
Shain, K. H., & Dalton, W. S. (2001). Cell adhesion is a key determinant in de novo multidrug resistance (MDR): new targets for the prevention of acquired MDR. Molecular Cancer Therapeutics, 1(1), 69–78.
Giussani, M., Merlino, G., Cappelletti, V., Tagliabue, E., & Daidone, M. G. (2015). Tumor-extracellular matrix interactions: identification of tools associated with breast cancer progression. Seminars in Cancer Biology, 35, 3–10. https://doi.org/10.1016/j.semcancer.2015.09.012.
Soon, P. S., Kim, E., Pon, C. K., Gill, A. J., Moore, K., Spillane, A. J., et al. (2013). Breast cancer-associated fibroblasts induce epithelial-to-mesenchymal transition in breast cancer cells. Endocrine-Related Cancer, 20(1), 1–12. https://doi.org/10.1530/erc-12-0227.
Gao, M. Q., Kim, B. G., Kang, S., Choi, Y. P., Park, H., Kang, K. S., et al. (2010). Stromal fibroblasts from the interface zone of human breast carcinomas induce an epithelial-mesenchymal transition-like state in breast cancer cells in vitro. Journal of Cell Science, 123(Pt 20), 3507–3514. https://doi.org/10.1242/jcs.072900.
Yuan, J., Liu, M., Yang, L., Tu, G., Zhu, Q., Chen, M., et al. (2015). Acquisition of epithelial-mesenchymal transition phenotype in the tamoxifen-resistant breast cancer cell: a new role for G protein-coupled estrogen receptor in mediating tamoxifen resistance through cancer-associated fibroblast-derived fibronectin and beta1-integrin signaling pathway in tumor cells. Breast Cancer Research, 17, 69. https://doi.org/10.1186/s13058-015-0579-y.
Amornsupak, K., Insawang, T., Thuwajit, P., O-Charoenrat, P., Eccles, S. A., & Thuwajit, C. (2014). Cancer-associated fibroblasts induce high mobility group box 1 and contribute to resistance to doxorubicin in breast cancer cells. BMC Cancer, 14, 955. https://doi.org/10.1186/1471-2407-14-955.
Huang, J., Ni, J., Liu, K., Yu, Y., Xie, M., Kang, R., et al. (2012). HMGB1 promotes drug resistance in osteosarcoma. Cancer Research, 72(1), 230–238. https://doi.org/10.1158/0008-5472.can-11-2001.
Boelens, M. C., Wu, T. J., Nabet, B. Y., Xu, B., Qiu, Y., Yoon, T., et al. (2014). Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell, 159(3), 499–513. https://doi.org/10.1016/j.cell.2014.09.051.
Cui, Q., Wang, B., Li, K., Sun, H., Hai, T., Zhang, Y., et al. (2018). Upregulating MMP-1 in carcinoma-associated fibroblasts reduces the efficacy of Taxotere on breast cancer synergized by Collagen IV. Oncology Letters, 16(3), 3537–3544. https://doi.org/10.3892/ol.2018.9092.
Landry, B. D., Leete, T., Richards, R., Cruz-Gordillo, P., Schwartz, H. R., Honeywell, M. E., et al. (2018). Tumor-stroma interactions differentially alter drug sensitivity based on the origin of stromal cells. Molecular Systems Biology, 14(8), e8322–10.15252/msb.20188322.
Marusyk, A., Tabassum, D. P., Janiszewska, M., Place, A. E., Trinh, A., Rozhok, A. I., et al. (2016). Spatial proximity to fibroblasts impacts molecular features and therapeutic sensitivity of breast cancer cells influencing clinical outcomes. Cancer Research, 76(22), 6495–6506. https://doi.org/10.1158/0008-5472.can-16-1457.
Senthebane, D. A., Rowe, A., Thomford, N. E., Shipanga, H., Munro, D., Al Mazeedi, M. A. M., et al. (2017). The role of tumor microenvironment in chemoresistance: to survive, keep your enemies closer. International Journal of Molecular Sciences, 18(7), 1586. https://doi.org/10.3390/ijms18071586.
Lin, C. H., Pelissier, F. A., Zhang, H., Lakins, J., Weaver, V. M., Park, C., et al. (2015). Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Molecular Biology of the Cell, 26(22), 3946–3953. https://doi.org/10.1091/mbc.E15-07-0456.
Ozdemir, B. C., Pentcheva-Hoang, T., Carstens, J. L., Zheng, X., Wu, C. C., Simpson, T. R., et al. (2015). Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell, 28(6), 831–833. https://doi.org/10.1016/j.ccell.2015.11.002.
Duyverman, A. M. M. J., Steller, E. J. A., Fukumura, D., Jain, R. K., & Duda, D. G. (2012). Studying primary tumor-associated fibroblast involvement in cancer metastasis in mice. Nature Protocols, 7(4), 756–762. https://doi.org/10.1038/nprot.2012.031.
Rhim, A. D., Oberstein, P. E., Thomas, D. H., Mirek, E. T., Palermo, C. F., Sastra, S. A., et al. (2014). Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell, 25(6), 735–747. https://doi.org/10.1016/j.ccr.2014.04.021.
Yauch, R. L., Gould, S. E., Scales, S. J., Tang, T., Tian, H., Ahn, C. P., et al. (2008). A paracrine requirement for hedgehog signalling in cancer. Nature, 455(7211), 406–410. https://doi.org/10.1038/nature07275.
Olive, K. P., Jacobetz, M. A., Davidson, C. J., Gopinathan, A., McIntyre, D., Honess, D., et al. (2009). Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science, 324(5933), 1457–1461. https://doi.org/10.1126/science.1171362.
Ko, A. H., LoConte, N., Tempero, M. A., Walker, E. J., Kate Kelley, R., Lewis, S., et al. (2016). A phase I study of FOLFIRINOX plus IPI-926, a hedgehog pathway inhibitor, for advanced pancreatic adenocarcinoma. Pancreas, 45(3), 370–375. https://doi.org/10.1097/mpa.0000000000000458.
Fearon, D. T. (2014). The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunology Research, 2(3), 187–193. https://doi.org/10.1158/2326-6066.cir-14-0002.
Kraman, M., Bambrough, P. J., Arnold, J. N., Roberts, E. W., Magiera, L., Jones, J. O., et al. (2010). Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science, 330(6005), 827–830. https://doi.org/10.1126/science.1195300.
Duperret, E. K., Trautz, A., Ammons, D., Perales-Puchalt, A., Wise, M. C., Yan, J., et al. (2018). Alteration of the tumor stroma using a consensus DNA vaccine targeting fibroblast activation protein (FAP) synergizes with antitumor vaccine therapy in mice. Clinical Cancer Research, 24(5), 1190–1201. https://doi.org/10.1158/1078-0432.ccr-17-2033.
Gottschalk, S., Yu, F., Ji, M., Kakarla, S., & Song, X. T. (2013). A vaccine that co-targets tumor cells and cancer associated fibroblasts results in enhanced antitumor activity by inducing antigen spreading. PLoS One, 8(12), e82658. https://doi.org/10.1371/journal.pone.0082658.
Loeffler, M., Kruger, J. A., Niethammer, A. G., & Reisfeld, R. A. (2006). Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. The Journal of Clinical Investigation, 116(7), 1955–1962. https://doi.org/10.1172/jci26532.
Meng, M., Wang, W., Yan, J., Tan, J., Liao, L., Shi, J., et al. (2016). Immunization of stromal cell targeting fibroblast activation protein providing immunotherapy to breast cancer mouse model. Tumour Biology, 37(8), 10317–10327. https://doi.org/10.1007/s13277-016-4825-4.
Ostermann, E., Garin-Chesa, P., Heider, K. H., Kalat, M., Lamche, H., Puri, C., et al. (2008). Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clinical Cancer Research, 14(14), 4584–4592. https://doi.org/10.1158/1078-0432.ccr-07-5211.
Femel, J., Huijbers, E. J., Saupe, F., Cedervall, J., Zhang, L., Roswall, P., et al. (2014). Therapeutic vaccination against fibronectin ED-A attenuates progression of metastatic breast cancer. Oncotarget, 5(23), 12418–12427. https://doi.org/10.18632/oncotarget.2628.
Park, C. Y., Min, K. N., Son, J. Y., Park, S. Y., Nam, J. S., Kim, D. K., et al. (2014). An novel inhibitor of TGF-beta type I receptor, IN-1130, blocks breast cancer lung metastasis through inhibition of epithelial-mesenchymal transition. Cancer Letters, 351(1), 72–80. https://doi.org/10.1016/j.canlet.2014.05.006.
Fang, Y., Chen, Y., Yu, L., Zheng, C., Qi, Y., Li, Z., et al. (2013). Inhibition of breast cancer metastases by a novel inhibitor of TGFbeta receptor 1. Journal of the National Cancer Institute, 105(1), 47–58. https://doi.org/10.1093/jnci/djs485.
Ehata, S., Hanyu, A., Fujime, M., Katsuno, Y., Fukunaga, E., Goto, K., et al. (2007). Ki26894, a novel transforming growth factor-beta type I receptor kinase inhibitor, inhibits in vitro invasion and in vivo bone metastasis of a human breast cancer cell line. Cancer Science, 98(1), 127–133. https://doi.org/10.1111/j.1349-7006.2006.00357.x.
Bandyopadhyay, A., Agyin, J. K., Wang, L., Tang, Y., Lei, X., Story, B. M., et al. (2006). Inhibition of pulmonary and skeletal metastasis by a transforming growth factor-β type I receptor kinase inhibitor. Cancer Research, 66(13), 6714–6721. https://doi.org/10.1158/0008-5472.can-05-3565.
Formenti, S. C., Lee, P., Adams, S., Goldberg, J. D., Li, X., Xie, M. W., et al. (2018). Focal irradiation and systemic TGFbeta blockade in metastatic breast cancer. Clinical Cancer Research, 24(11), 2493–2504. https://doi.org/10.1158/1078-0432.ccr-17-3322.
Giaccone, G., Bazhenova, L. A., Nemunaitis, J., Tan, M., Juhasz, E., Ramlau, R., et al. (2015). A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. European Journal of Cancer, 51(16), 2321–2329. https://doi.org/10.1016/j.ejca.2015.07.035.
Xiang, J., Hurchla, M. A., Fontana, F., Su, X., Amend, S. R., Esser, A. K., et al. (2015). CXCR4 protein epitope mimetic antagonist POL5551 disrupts metastasis and enhances chemotherapy effect in triple-negative breast cancer. Molecular Cancer Therapeutics, 14(11), 2473–2485. https://doi.org/10.1158/1535-7163.mct-15-0252.
Peng, S. B., Zhang, X., Paul, D., Kays, L. M., Gough, W., Stewart, J., et al. (2015). Identification of LY2510924, a novel cyclic peptide CXCR4 antagonist that exhibits antitumor activities in solid tumor and breast cancer metastatic models. Molecular Cancer Therapeutics, 14(2), 480–490. https://doi.org/10.1158/1535-7163.mct-14-0850.
Ling, X., Spaeth, E., Chen, Y., Shi, Y., Zhang, W., Schober, W., et al. (2013). The CXCR4 antagonist AMD3465 regulates oncogenic signaling and invasiveness in vitro and prevents breast cancer growth and metastasis in vivo. PLoS One, 8(3), e58426. https://doi.org/10.1371/journal.pone.0058426.
Galsky, M. D., Vogelzang, N. J., Conkling, P., Raddad, E., Polzer, J., Roberson, S., et al. (2014). A phase I trial of LY2510924, a CXCR4 peptide antagonist, in patients with advanced cancer. Clinical Cancer Research, 20(13), 3581–3588. https://doi.org/10.1158/1078-0432.ccr-13-2686.
Hainsworth, J. D., Reeves, J. A., Mace, J. R., Crane, E. J., Hamid, O., Stille, J. R., et al. (2016). A randomized, open-label phase 2 study of the CXCR4 inhibitor LY2510924 in combination with sunitinib versus sunitinib alone in patients with metastatic renal cell carcinoma (RCC). Targeted Oncology, 11(5), 643–653. https://doi.org/10.1007/s11523-016-0434-9.
Salgia, R., Stille, J. R., Weaver, R. W., McCleod, M., Hamid, O., Polzer, J., et al. (2017). A randomized phase II study of LY2510924 and carboplatin/etoposide versus carboplatin/etoposide in extensive-disease small cell lung cancer. Lung Cancer, 105, 7–13. https://doi.org/10.1016/j.lungcan.2016.12.020.
Loktev, A., Lindner, T., Mier, W., Debus, J., Altmann, A., Jager, D., et al. (2018). A tumor-imaging method targeting cancer-associated fibroblasts. Journal of Nuclear Medicine, 59(9), 1423–1429. https://doi.org/10.2967/jnumed.118.210435.
Zhou, Z., Qutaish, M., Han, Z., Schur, R. M., Liu, Y., Wilson, D. L., et al. (2015). MRI detection of breast cancer micrometastases with a fibronectin-targeting contrast agent. Nature Communications, 6, 7984. https://doi.org/10.1038/ncomms8984.
Butsch, V., Borgel, F., Galla, F., Schwegmann, K., Hermann, S., Schafers, M., et al. (2018). Design, (radio)synthesis, and in vitro and in vivo evaluation of highly selective and potent matrix metalloproteinase 12 (MMP-12) inhibitors as radiotracers for positron emission tomography. Journal of Medicinal Chemistry, 61(9), 4115–4134. https://doi.org/10.1021/acs.jmedchem.8b00200.
Matusiak, N., Castelli, R., Tuin, A. W., Overkleeft, H. S., Wisastra, R., Dekker, F. J., et al. (2015). A dual inhibitor of matrix metalloproteinases and a disintegrin and metalloproteinases, [(1)(8)F]FB-ML5, as a molecular probe for non-invasive MMP/ADAM-targeted imaging. Bioorganic & Medicinal Chemistry, 23(1), 192–202. https://doi.org/10.1016/j.bmc.2014.11.013.
Matusiak, N., van Waarde, A., Bischoff, R., Oltenfreiter, R., van de Wiele, C., Dierckx, R. A., et al. (2013). Probes for non-invasive matrix metalloproteinase-targeted imaging with PET and SPECT. Current Pharmaceutical Design, 19(25), 4647–4672.
Wagner, S., Breyholz, H. J., Faust, A., Holtke, C., Levkau, B., Schober, O., et al. (2006). Molecular imaging of matrix metalloproteinases in vivo using small molecule inhibitors for SPECT and PET. Current Medicinal Chemistry, 13(23), 2819–2838.
Xu, K., Rajagopal, S., Klebba, I., Dong, S., Ji, Y., Liu, J., et al. (2010). The role of fibroblast Tiam1 in tumor cell invasion and metastasis. Oncogene, 29(50), 6533–6542. https://doi.org/10.1038/onc.2010.385.
Chang, P. H., Hwang-Verslues, W. W., Chang, Y. C., Chen, C. C., Hsiao, M., Jeng, Y. M., et al. (2012). Activation of Robo1 signaling of breast cancer cells by Slit2 from stromal fibroblast restrains tumorigenesis via blocking PI3K/Akt/beta-catenin pathway. Cancer Research, 72(18), 4652–4661. https://doi.org/10.1158/0008-5472.can-12-0877.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Houthuijzen, J.M., Jonkers, J. Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment. Cancer Metastasis Rev 37, 577–597 (2018). https://doi.org/10.1007/s10555-018-9768-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-018-9768-3