Abstract
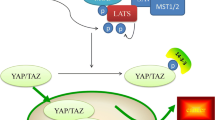
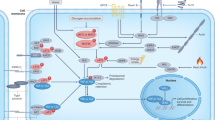
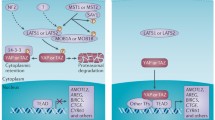
The major functions of Hippo (Hpo) signaling pathway are to control cell growth, proliferation, and apoptosis. As its important downstream player, yes-associated protein (YAP)-1 was originally found to promote cell proliferation and transformation. Overexpression of YAP-1 has been linked to tumor progression and worse survival in certain malignancies. However, it has been recently recognized as a tumor suppressor gene as well since it also induces apoptosis. Decreased or absent expression of YAP-1 is highly correlated with tumor progression and worse survival in other tumors such as breast cancer. It is clear that YAP-1 plays a dual role as oncogene and tumor suppressor gene in human oncogenesis, depending on the specific tissue type involved. Here, we reviewed the recent research on both the oncogenic and tumor suppressor function of YAP-1 and its significance in human malignancy. The clinical implication of YAP-1 expression in cancer prognosis and the development of targeted therapy will also be discussed.



Similar content being viewed by others
References
Zhao, B., et al. (2013). The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nature Cell Biology, 13, 877–883.
Zhou, D., et al. (2011). Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proceedings of the National Academy of Sciences of the United States of America, 108, E1312–E1320.
Tamm, C., et al. (2011). Regulation of mouse embryonic stem cell self-renewal by a Yes-YAP-TEAD2 signaling pathway downstream of LIF. Journal of Cell Science, 124, 1136–1144.
Zhang, H., et al. (2011). Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proceedings of the National Academy of Sciences of the United States of America, 108, 2270–2275.
Yu, F. X., et al. (2012). Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell, 150(4), 780–791.
Zhao, B., et al. (2007). Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes and Development, 21, 2747–2761.
Lapi, E., et al. (2008). PML, YAP, and p73 are components of a proapoptotic autoregulatory feedback loop. Molecular Cell, 32, 803–814.
Strano, S., et al. (2007). The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA damage. Molecular Cell, 18, 447–459.
Yuan, M., et al. (2008). Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death and Differentiation, 15, 1752–1759.
Bertini, E. (2009). YAP: at the crossroad between transformation and tumor suppression. Cell Cycle, 8, 49–57.
Zender, L., et al. (2006). Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell, 125, 1253–1267.
Overholtzer, M., et al. (2006). Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proceedings of the National Academy of Sciences of the United States of America, 103, 12405–12410.
Zhao, B., et al. (2008). TEAD mediates YAP-dependent gene induction and growth control. Genes and Development, 22, 1962–1971.
Barry, E. R., et al. (2013). Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature, 493, 106–110.
Hao, Y., et al. (2008). Tumor suppressor LATS1 is a negative regulator of oncogene YAP. Journal of Biological Chemistry, 283, 5496–5509.
Oka, T., et al. (2008). Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP). Journal of Biological Chemistry, 283, 27534–27546.
Ren, F. L., et al. (2010). Hippo signaling regulates Yorkie nuclear localization and activity through 14-3-3 dependent and independent mechanisms. Developmental Biology, 337, 303–312.
Zhao, B., et al. (2010). A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes and Development, 24, 72–85.
Basu, S., et al. (2003). Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Molecular Cell, 11, 11–23.
Zhao, B., et al. (2011). Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes and Development, 25, 51–63.
Oka, T., et al. (2010). Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. The Biochemical Journal, 432, 461–472.
Remue, E., et al. (2010). TAZ interacts with zonula occludens-1 and -2 proteins in a PDZ-1 dependent manner. FEBS Letters, 584, 4175–4180.
Wu, S., et al. (2003). Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell, 114, 445–456.
Harvey, K. F., et al. (2003). The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell, 114, 457–467.
Callus, B. A., et al. (2006). Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. The FEBS Journal, 273, 4264–4276.
Chan, E. H., et al. (2005). Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene, 24, 2076–2086.
Praskova, M., et al. (2008). MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Current Biology: CB, 18, 311–321.
Oka, T. V., et al. (2008). Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP). Journal of Biological Chemistry, 283, 27534–27546.
Zhang, L., et al. (2008). The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Developmental Cell, 14, 377–387.
Wu, S., et al. (2008). The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Developmental Cell, 14(2008), 388–398.
Ziosi, M., et al. (2010). dMyc functions downstream of Yorkie to promote the supercompetitive behavior of hippo pathway mutant cells. PLoS Genetics, 6(9), e1001140. doi:10.1371/journal.pgen.1001140.
Neto-Silva, R. M., et al. (2010). Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Developmental Cell, 19, 507–520.
Zhang, J., et al. (2009). YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nature Cell Biology, 11, 1444–1450.
Alarcon, C., et al. (2009). Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell, 139, 757–769.
Vitolo, M. I., et al. (2007). The RUNX2 transcription factor cooperates with the YES-associated protein, YAP65, to promote cell transformation. Cancer Biology & Therapy, 6, 856–863.
Komuro, A., et al. (2003). WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. Journal of Biological Chemistry, 278, 33334–33341.
Strano, S., et al. (2001). Physical interaction with Yes-associated protein enhances p73 transcriptional activity. Journal of Biological Chemistry, 276, 15164–15173.
Lamar, J. M., et al. (2012). The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proceedings of the National Academy of Sciences of the United States of America, 109, E2441–E2450.
Xu, M. Z., et al. (2011). AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene, 30, 1229–1240.
Ehsanian, R., et al. (2010). YAP dysregulation by phosphorylation or DeltaNp63-mediated gene repression promotes proliferation, survival and migration in head and neck cancer subsets. Oncogene, 29, 6160–6171.
Yu,S.J., et al. (2013). MicroRNA-200a promotes anoikis resistance and metastasis by targeting YAP1 in human breast cancer. Clinical Cancer Research, 19, 1389–1399.
Espanel, X., et al. (2001). Yes-associated protein and p53-binding protein-2 interact through their WW and SH3 domains. Journal of Biological Chemistry, 276, 14514–14523.
Danovi, S. A., et al. (2008). Yes-associated protein (YAP) is a critical mediator of c-Jun-dependent apoptosis. Cell Death and Differentiation, 15, 217–219.
Levy, D., et al. (2008). Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Molecular Cell, 29, 350–361.
Levy, D., et al. (2007). The Yes-associated protein 1 stabilizes p73 by preventing Itch-mediated ubiquitination of p73. Cell Death and Differentiation, 14, 743–751.
Okazaki, T., et al. (2012). Up-regulation of endogenous PML induced by a combination of interferon-beta and temozolomide enhances p73/YAP-mediated apoptosis in glioblastoma. Cancer Letters, 323, 199–207.
Yee, K.S., et al. (2012). A RASSF1A polymorphism restricts p53/p73 activation and associates with poor survival and accelerated age of onset of soft tissue sarcoma. Cancer Research, 72, 2206–2217.
Matallanas, D., et al. (2007). RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Molecular Cell, 27, 962–975.
Camargo, F. D., et al. (2007). YAP1 increases organ size and expands undifferentiated progenitor cells. Current Biology, 17(23), 2054–2060.
Lian, I., et al. (2010). The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes & Development, 24(11), 1106–1118.
Zhou, D., et al. (2011). Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proceedings of the National Academy of Sciences of the United States of America, 108(49), E1312–E1320.
Varelas, X., et al. (2010). The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Developmental Cell, 19, 831–844.
Yi, C., et al. (2010). Merlin in organ size control and tumorigenesis: hippo versus EGFR? Genes and Development, 24, 1673–1679.
Fan, R., et al. (2013). Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proceedings of the National Academy of Sciences of the United States of America, 110(7), 2569–2574.
Fernandez, L. A., et al. (2009). YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes and Development, 23, 2729–2741.
Tumaneng, K., et al. (2012). YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nature Cell Biology, 14, 1322–1329.
Rosenbluh, J., et al. (2012). â-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell, 151(7), 1457–1473.
Tschaharganeh, D. F., et al. (2013). Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology, 144(7), 1530–1542.
Muramatsu, T., et al. (2011). YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis, 32, 389–398.
Imoto, I., et al. (2001). Identification of cIAP1 as a candidate target gene within an amplicon at 11q22 in esophageal squamous cell carcinomas. Cancer Research, 61, 6629–6634.
Liu, T., et al. (2013). Clinical significance of yes-associated protein overexpression in cervical carcinoma: the differential effects based on histotypes. International Journal of Gynecological Cancer, 23, 735–742.
Tang, S.C., et al. (2002). BAG-1, an anti-apoptotic tumour marker. IUBMB Life, 53, 99–105.
Tang, S. C., et al. (1999). Expression of BAG-1 in invasive breast carcinomas. Journal of Clinical Oncology, 17, 1710–1719.
Turner, B. C., et al. (2001). BAG-1: a novel biomarker predicting long-term survival in early-stage breast cancer. Journal of Clinical Oncology, 19, 992–1000.
Xu, M. Z., et al. (2009). Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer, 115, 4576–4585.
Song, M., et al. (2012). Nuclear expression of Yes-associated protein 1 correlates with poor prognosis in intestinal type gastric cancer. Anticancer Research, 32, 3827–3834.
Wang, Y., et al. (2010). Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Science, 101, 1279–1285.
Su, L. L., et al. (2012). Expression of Yes-associated protein in non-small cell lung cancer and its relationship with clinical pathological factors. Chinese Medical Journal, 125, 4003–4008.
Carter, S. L., et al. (1994). Loss of heterozygosity at 11q22-q23 in breast cancer. Cancer Research, 54, 6270–6274.
Winqvist, R., et al. (1995). Loss of heterozygosity for chromosome 11 in primary human breast tumors is associated with poor survival after metastasis. Cancer Research, 55, 2660–2664.
Gudmundsson, J., et al. (1995). Loss of heterozygosity at chromosome 11 in breast cancer: association of prognostic factors with genetic alterations. British Journal of Cancer, 72, 696–701.
Hampton, G. M., et al. (1994). Loss of heterozygosity in sporadic human breast carcinoma: a common region between 11q22 and 11q23.3. Cancer Research, 54, 4586–4589.
Tomlinson, I. P., et al. (1995). Loss of heterozygosity on chromosome 11 q in breast cancer. Journal of Clinical Pathology, 48, 424–428.
Tufail, R., et al. (2012). Loss of Yes-associated protein (YAP) expression is associated with estrogen and progesterone receptors negativity in invasive breast carcinomas. Breast Cancer Research and Treatment, 131, 743–750.
Wang, Y., et al. (2013). Clinical and prognostic significance of Yes-associated protein in colorectal cancer. Tumour Biology, 34(4), 2169–2174.
Fujii, M., et al. (2012). Exploration of a new drug that targets YAP. Journal of Biochemistry, 152(3), 209–211.
Bao, Y., et al. (2011). A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. Journal of Biochemistry, 150(2), 199–208.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hai Wang and Yu-Chen Du made equal contribution to the manuscript.
Rights and permissions
About this article
Cite this article
Wang, H., Du, YC., Zhou, Xj. et al. The dual functions of YAP-1 to promote and inhibit cell growth in human malignancy. Cancer Metastasis Rev 33, 173–181 (2014). https://doi.org/10.1007/s10555-013-9463-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-013-9463-3