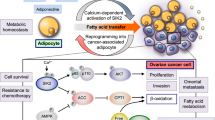
Abstract
Ovarian adenocarcinoma is characterized by a late detection, dissemination of cancer cells into the whole peritoneum, and the frequent acquisition of chemoresistance. If these particularities can be explained in part by intrinsic properties of ovarian cancer cells, an increased number of studies show the importance of the tumor microenvironment in tumor progression. Ovarian cancer cells can regulate the composition of their stroma in promoting the formation of ascitic fluid, rich in cytokines and bioactive lipids, and in stimulating the differentiation of stromal cells into a pro-tumoral phenotype. In return, cancer-associated fibroblasts, cancer-associated mesenchymal stem cells, tumor-associated macrophages, or other peritoneal cells, such as adipocytes and mesothelial cells can regulate tumor growth, angiogenesis, dissemination, and chemoresistance. This review focuses on the current knowledge about the roles of stromal cells and the associated secreted factors on tumor progression. We also summarize the different studies showing that targeting the microenvironment represents a great potential for improving the prognosis of patients with ovarian adenocarcinoma.



Similar content being viewed by others
References
Colombo, N., Van, G. T., Parma, G., Amant, F., Gatta, G., Sessa, C., et al. (2006). Ovarian Cancer Critical Reviews Oncology/Hematology, 60(2), 159–179.
Hennessy, B. T., Coleman, R. L., & Markman, M. (2009). Ovarian cancer. Lancet, 374(9698), 1371–1382.
Romero, I., & Bast, R. C., Jr. (2012). Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy. Endocrinology, 153(4), 1593–1602.
Vaughan, S., Coward, J. I., Bast, R. C., Jr., Berchuck, A., Berek, J. S., Brenton, J. D., et al. (2011). Rethinking ovarian cancer: recommendations for improving outcomes. Nature Reviews Cancer, 11(10), 719–725.
Shih, I., & Kurman, R. J. (2004). Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. The American Journal of Pathology, 164(5), 1511–1518.
Cheng, B., Lu, W., Xiaoyun, W., YaXia, C., & Xie, X. (2009). Extra-abdominal metastases from epithelial ovarian carcinoma: an analysis of 20 cases. International Journal of Gynecological Cancer, 19(4), 611–614.
Sekine, M., Yoshihara, K., Komata, D., Haino, K., Nishino, K., & Tanaka, K. (2013). Increased incidence of brain metastases in BRCA1-related ovarian cancers. The Journal of Obstetrics and Gynaecology Research, 39(1), 292–296.
Ziegler, J., Gliedman, P., Fass, D., Beckman, M., Neophytides, A., & Steinfeld, A. (1987). Brain metastases from ovarian cancer. Journal of Neuro-Oncology, 5(3), 211–215.
Zhou, J. J., & Miao, X. Y. (2012). Gastric metastasis from ovarian carcinoma: a case report and literature review. World Journal of Gastroenterology, 18(43), 6341–6344.
Castells, M., Thibault, B., Delord, J. P., & Couderc, B. (2012). Implication of tumor microenvironment in chemoresistance: tumor-associated stromal cells protect tumor cells from cell death. International Journal of Molecular Sciences, 13(8), 9545–9571.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144(5), 646–674.
Hanahan, D., & Coussens, L. M. (2012). Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell, 21(3), 309–322.
Touboul, C., Lis, R., Al, F. H., Raynaud, C. M., Warfa, M., Althawadi, H., et al. (2013). Mesenchymal stem cells enhance ovarian cancer cell infiltration through IL6 secretion in an amniochorionic membrane based 3D model. Journal of Translational Medicine, 11(1), 28.
Rieppi, M., Vergani, V., Gatto, C., Zanetta, G., Allavena, P., Taraboletti, G., et al. (1999). Mesothelial cells induce the motility of human ovarian carcinoma cells. International Journal of Cancer, 80(2), 303–307.
Wang, E., Ngalame, Y., Panelli, M. C., Nguyen-Jackson, H., Deavers, M., Mueller, P., et al. (2005). Peritoneal and subperitoneal stroma may facilitate regional spread of ovarian cancer. Clinical Cancer Research, 11(1), 113–122.
Hollingsworth, H. C., Kohn, E. C., Steinberg, S. M., Rothenberg, M. L., & Merino, M. J. (1995). Tumor angiogenesis in advanced stage ovarian carcinoma. The American Journal of Pathology, 147(1), 33–41.
Duncan, T. J., Al-Attar, A., Rolland, P., Scott, I. V., Deen, S., Liu, D. T., et al. (2008). Vascular endothelial growth factor expression in ovarian cancer: a model for targeted use of novel therapies? Clinical Cancer Research, 14(10), 3030–3035.
Byrne, A. T., Ross, L., Holash, J., Nakanishi, M., Hu, L., Hofmann, J. I., et al. (2003). Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clinical Cancer Research, 9(15), 5721–5728.
Malek, J. A., Martinez, A., Mery, E., Ferron, G., Huang, R., Raynaud, C., et al. (2012). Gene expression analysis of matched ovarian primary tumors and peritoneal metastasis. Journal of Translational Medicine, 10, 121.
Moradi, M. M., Carson, L. F., Weinberg, B., Haney, A. F., Twiggs, L. B., & Ramakrishnan, S. (1993). Serum and ascitic fluid levels of interleukin-1, interleukin-6, and tumor necrosis factor-alpha in patients with ovarian epithelial cancer. Cancer, 72(8), 2433–2440.
Xu, Y., Gaudette, D. C., Boynton, J. D., Frankel, A., Fang, X. J., Sharma, A., et al. (1995). Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clinical Cancer Research, 1(10), 1223–1232.
Xu, Y., Shen, Z., Wiper, D. W., Wu, M., Morton, R. E., Elson, P., et al. (1998). Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA, 280(8), 719–723.
Xu, Y., Fang, X. J., Casey, G., & Mills, G. B. (1995). Lysophospholipids activate ovarian and breast cancer cells. Biochemical Journal, 309(Pt 3), 933–940.
Yang, K., Zheng, D., Deng, X., Bai, L., Xu, Y., & Cong, Y. S. (2008). Lysophosphatidic acid activates telomerase in ovarian cancer cells through hypoxia-inducible factor-1alpha and the PI3K pathway. Journal of Cellular Biochemistry, 105(5), 1194–1201.
So, J., Navari, J., Wang, F. Q., & Fishman, D. A. (2004). Lysophosphatidic acid enhances epithelial ovarian carcinoma invasion through the increased expression of interleukin-8. Gynecologic Oncology, 95(2), 314–322.
Schwartz, B. M., Hong, G., Morrison, B. H., Wu, W., Baudhuin, L. M., Xiao, Y. J., et al. (2001). Lysophospholipids increase interleukin-8 expression in ovarian cancer cells. Gynecologic Oncology, 81(2), 291–300.
Fang, X., Schummer, M., Mao, M., Yu, S., Tabassam, F. H., Swaby, R., et al. (2002). Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochimica et Biophysica Acta, 1582(1–3), 257–264.
Fang, X., Yu, S., Bast, R. C., Liu, S., Xu, H. J., Hu, S. X., et al. (2004). Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. The Journal of Biological Chemistry, 279(10), 9653–9661.
Hu, Y. L., Tee, M. K., Goetzl, E. J., Auersperg, N., Mills, G. B., Ferrara, N., et al. (2001). Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. Journal of the National Cancer Institute, 93(10), 762–768.
Fishman, D. A., Liu, Y., Ellerbroek, S. M., & Stack, M. S. (2001). Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Research, 61(7), 3194–3199.
Said, N. A., Elmarakby, A. A., Imig, J. D., Fulton, D. J., & Motamed, K. (2008). SPARC ameliorates ovarian cancer-associated inflammation. Neoplasia, 10(10), 1092–1104.
Sengupta, S., Kim, K. S., Berk, M. P., Oates, R., Escobar, P., Belinson, J., et al. (2007). Lysophosphatidic acid downregulates tissue inhibitor of metalloproteinases, which are negatively involved in lysophosphatidic acid-induced cell invasion. Oncogene, 26(20), 2894–2901.
Li, H., Wang, D., Zhang, H., Kirmani, K., Zhao, Z., Steinmetz, R., et al. (2009). Lysophosphatidic acid stimulates cell migration, invasion, and colony formation as well as tumorigenesis/metastasis of mouse ovarian cancer in immunocompetent mice. Molecular Cancer Therapeutics, 8(6), 1692–1701.
Wang, G. L., Wen, Z. Q., Xu, W. P., Wang, Z. Y., Du, X. L., & Wang, F. (2008). Inhibition of lysophosphatidic acid receptor-2 expression by RNA interference decreases lysophosphatidic acid-induced urokinase plasminogen activator activation, cell invasion, and migration in ovarian cancer SKOV-3 cells. Croatian Medical Journal, 49(2), 175–181.
Wang, C., Michener, C. M., Belinson, J. L., Vaziri, S., Ganapathi, R., & Sengupta, S. (2010). Role of the 18:1 lysophosphatidic acid-ovarian cancer immunoreactive antigen domain containing 1 (OCIAD1)-integrin axis in generating late-stage ovarian cancer. Molecular Cancer Therapeutics, 9(6), 1709–1718.
Sengupta, S., Michener, C. M., Escobar, P., Belinson, J., & Ganapathi, R. (2008). Ovarian cancer immuno-reactive antigen domain containing 1 (OCIAD1), a key player in ovarian cancer cell adhesion. Gynecologic Oncology, 109(2), 226–233.
Frankel, A., & Mills, G. B. (1996). Peptide and lipid growth factors decrease cis-diamminedichloroplatinum-induced cell death in human ovarian cancer cells. Clinical Cancer Research, 2(8), 1307–1313.
Hong, G., Baudhuin, L. M., & Xu, Y. (1999). Sphingosine-1-phosphate modulates growth and adhesion of ovarian cancer cells. FEBS Letters, 460(3), 513–518.
Smicun, Y., Reierstad, S., Wang, F. Q., Lee, C., & Fishman, D. A. (2006). S1P regulation of ovarian carcinoma invasiveness. Gynecologic Oncology, 103(3), 952–959.
Devine, K. M., Smicun, Y., Hope, J. M., & Fishman, D. A. (2008). S1P induced changes in epithelial ovarian cancer proteolysis, invasion, and attachment are mediated by Gi and Rac. Gynecologic Oncology, 110(2), 237–245.
Park, K. S., Kim, M. K., Lee, H. Y., Kim, S. D., Lee, S. Y., Kim, J. M., et al. (2007). S1P stimulates chemotactic migration and invasion in OVCAR3 ovarian cancer cells. Biochemical and Biophysical Research Communications, 356(1), 239–244.
Ali-Fehmi, R., Morris, R. T., Bandyopadhyay, S., Che, M., Schimp, V., Malone, J. M., Jr., et al. (2005). Expression of cyclooxygenase-2 in advanced stage ovarian serous carcinoma: correlation with tumor cell proliferation, apoptosis, angiogenesis, and survival. American Journal of Obstetrics and Gynecology, 192(3), 819–825.
Ferrandina, G., Lauriola, L., Distefano, M. G., Zannoni, G. F., Gessi, M., Legge, F., et al. (2002). Increased cyclooxygenase-2 expression is associated with chemotherapy resistance and poor survival in cervical cancer patients. Journal of Clinical Oncology, 20(4), 973–981.
Munkarah, A. R., Morris, R., Baumann, P., Deppe, G., Malone, J., Diamond, M. P., et al. (2002). Effects of prostaglandin E(2) on proliferation and apoptosis of epithelial ovarian cancer cells. Journal of the Society for Gynecologic Investigation, 9(3), 168–173.
Rask, K., Zhu, Y., Wang, W., Hedin, L., & Sundfeldt, K. (2006). Ovarian epithelial cancer: a role for PGE2-synthesis and signalling in malignant transformation and progression. Molecular Cancer, 5, 62.
Obermajer, N., Muthuswamy, R., Odunsi, K., Edwards, R. P., & Kalinski, P. (2011). PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Research, 71(24), 7463–7470.
Naka, T., Nishimoto, N., & Kishimoto, T. (2002). The paradigm of IL-6: from basic science to medicine. Arthritis Research, 4(Suppl 3), S233–S242.
Nash, M. A., Ferrandina, G., Gordinier, M., Loercher, A., & Freedman, R. S. (1999). The role of cytokines in both the normal and malignant ovary. Endocrine-Related Cancer, 6(1), 93–107.
Nilsson, M. B., Langley, R. R., & Fidler, I. J. (2005). Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Research, 65(23), 10794–10800.
Garg, R., Wollan, M., Galic, V., Garcia, R., Goff, B. A., Gray, H. J., et al. (2006). Common polymorphism in interleukin 6 influences survival of women with ovarian and peritoneal carcinoma. Gynecologic Oncology, 103(3), 793–796.
Plante, M., Rubin, S. C., Wong, G. Y., Federici, M. G., Finstad, C. L., & Gastl, G. A. (1994). Interleukin-6 level in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. Cancer, 73(7), 1882–1888.
Tempfer, C., Zeisler, H., Sliutz, G., Haeusler, G., Hanzal, E., & Kainz, C. (1997). Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecologic Oncology, 66(1), 27–30.
Penson, R. T., Kronish, K., Duan, Z., Feller, A. J., Stark, P., Cook, S. E., et al. (2000). Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. International Journal of Gynecological Cancer, 10(1), 33–41.
Scambia, G., Testa, U., Benedetti, P. P., Foti, E., Martucci, R., Gadducci, A., et al. (1995). Prognostic significance of interleukin 6 serum levels in patients with ovarian cancer. British Journal of Cancer, 71(2), 354–356.
Wang, Y., Niu, X. L., Qu, Y., Wu, J., Zhu, Y. Q., Sun, W. J., et al. (2010). Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Letters, 295(1), 110–123.
Syed, V., Ulinski, G., Mok, S. C., & Ho, S. M. (2002). Reproductive hormone-induced, STAT3-mediated interleukin 6 action in normal and malignant human ovarian surface epithelial cells. Journal of the National Cancer Institute, 94(8), 617–629.
Obata, N. H., Tamakoshi, K., Shibata, K., Kikkawa, F., & Tomoda, Y. (1997). Effects of interleukin-6 on in vitro cell attachment, migration and invasion of human ovarian carcinoma. Anticancer Research, 17(1A), 337–342.
Rath, K. S., Funk, H. M., Bowling, M. C., Richards, W. E., & Drew, A. F. (2010). Expression of soluble interleukin-6 receptor in malignant ovarian tissue. American Journal of Obstetrics and Gynecology, 203(3), 230–238.
Waugh, D. J., & Wilson, C. (2008). The interleukin-8 pathway in cancer. Clinical Cancer Research, 14(21), 6735–6741.
Xu, L., & Fidler, I. J. (2000). Interleukin 8: an autocrine growth factor for human ovarian cancer. Oncology Research, 12(2), 97–106.
Merritt, W. M., Lin, Y. G., Spannuth, W. A., Fletcher, M. S., Kamat, A. A., Han, L. Y., et al. (2008). Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. Journal of the National Cancer Institute, 100(5), 359–372.
Shahzad, M. M., Arevalo, J. M., Armaiz Pena, G. N., Lu, C., Stone, R. L., Moreno-Smith, M., et al (2010). Stress effects on FOSB and interleukin-8 (IL8) driven ovarian cancer growth and metastasis. J Biol Chem
Kassim, S. K., El Salahy, E. M., Fayed, S. T., Helal, S. A., Helal, T., Azzam, E., et al. (2004). Vascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patients. Clinical Biochemistry, 37(5), 363–369.
Huang, S., Robinson, J. B., Deguzman, A., Bucana, C. D., & Fidler, I. J. (2000). Blockade of nuclear factor-kappaB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Research, 60(19), 5334–5339.
Lee, L. F., Hellendall, R. P., Wang, Y., Haskill, J. S., Mukaida, N., Matsushima, K., et al. (2000). IL-8 reduced tumorigenicity of human ovarian cancer in vivo due to neutrophil infiltration. Journal of Immunology, 164(5), 2769–2775.
Ferrara, N., Gerber, H. P., & LeCouter, J. (2003). The biology of VEGF and its receptors. Nature Medicine, 9(6), 669–676.
Kryczek, I., Lange, A., Mottram, P., Alvarez, X., Cheng, P., Hogan, M., et al. (2005). CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Research, 65(2), 465–472.
Mesiano, S., Ferrara, N., & Jaffe, R. B. (1998). Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. The American Journal of Pathology, 153(4), 1249–1256.
Hu, L., Hofmann, J., Zaloudek, C., Ferrara, N., Hamilton, T., & Jaffe, R. B. (2002). Vascular endothelial growth factor immunoneutralization plus Paclitaxel markedly reduces tumor burden and ascites in athymic mouse model of ovarian cancer. The American Journal of Pathology, 161(5), 1917–1924.
Hu, L., Hofmann, J., Holash, J., Yancopoulos, G. D., Sood, A. K., & Jaffe, R. B. (2005). Vascular endothelial growth factor trap combined with paclitaxel strikingly inhibits tumor and ascites, prolonging survival in a human ovarian cancer model. Clinical Cancer Research, 11(19 Pt 1), 6966–6971.
Heitz, F., Harter, P., Barinoff, J., Beutel, B., Kannisto, P., Grabowski, J. P., et al. (2012). Bevacizumab in the treatment of ovarian cancer. Advances in Therapy, 29(9), 723–735.
Scotton, C. J., Wilson, J. L., Scott, K., Stamp, G., Wilbanks, G. D., Fricker, S., et al. (2002). Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Research, 62(20), 5930–5938.
Kajiyama, H., Shibata, K., Terauchi, M., Ino, K., Nawa, A., & Kikkawa, F. (2008). Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. International Journal of Cancer, 122(1), 91–99.
Psyrri, A., Kassar, M., Yu, Z., Bamias, A., Weinberger, P. M., Markakis, S., et al. (2005). Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clinical Cancer Research, 11(24 Pt 1), 8637–8643.
Gui, T. and Shen, K. (2012). The epidermal growth factor receptor as a therapeutic target in epithelial ovarian cancer. Cancer Epidemiol
Schilder, R. J., Pathak, H. B., Lokshin, A. E., Holloway, R. W., Alvarez, R. D., Aghajanian, C., et al. (2009). Phase II trial of single agent cetuximab in patients with persistent or recurrent epithelial ovarian or primary peritoneal carcinoma with the potential for dose escalation to rash. Gynecologic Oncology, 113(1), 21–27.
Ahmed, N., Maines-Bandiera, S., Quinn, M. A., Unger, W. G., Dedhar, S., & Auersperg, N. (2006). Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. American Journal of Physiology. Cell Physiology, 290(6), C1532–C1542.
Colomiere, M., Ward, A. C., Riley, C., Trenerry, M. K., Cameron-Smith, D., Findlay, J., et al. (2009). Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. British Journal of Cancer, 100(1), 134–144.
Balkwill, F. (2009). Tumour necrosis factor and cancer. Nature Reviews Cancer, 9(5), 361–371.
Naylor, M. S., Stamp, G. W., Foulkes, W. D., Eccles, D., & Balkwill, F. R. (1993). Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. Journal Clinical Investigation, 91(5), 2194–2206.
Kulbe, H., Thompson, R., Wilson, J. L., Robinson, S., Hagemann, T., Fatah, R., et al. (2007). The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Research, 67(2), 585–592.
Charles, K. A., Kulbe, H., Soper, R., Escorcio-Correia, M., Lawrence, T., Schultheis, A., et al. (2009). The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. The Journal of Clinical Investigation, 119(10), 3011–3023.
Elliott, R. L., & Blobe, G. C. (2005). Role of transforming growth factor Beta in human cancer. Journal of Clinical Oncology, 23(9), 2078–2093.
Nakanishi, Y., Kodama, J., Yoshinouchi, M., Tokumo, K., Kamimura, S., Okuda, H., et al. (1997). The expression of vascular endothelial growth factor and transforming growth factor-beta associates with angiogenesis in epithelial ovarian cancer. International Journal of Gynecological Pathology, 16(3), 256–262.
Rodriguez, G. C., Haisley, C., Hurteau, J., Moser, T. L., Whitaker, R., Bast, R. C., Jr., et al. (2001). Regulation of invasion of epithelial ovarian cancer by transforming growth factor-beta. Gynecologic Oncology, 80(2), 245–253.
Thery, C., Zitvogel, L., & Amigorena, S. (2002). Exosomes: composition, biogenesis and function. Nature Reviews Immunology, 2(8), 569–579.
Peng, P., Yan, Y., & Keng, S. (2011). Exosomes in the ascites of ovarian cancer patients: origin and effects on anti-tumor immunity. Oncology Reports, 25(3), 749–762.
Keller, S., Konig, A. K., Marme, F., Runz, S., Wolterink, S., Koensgen, D., et al. (2009). Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Letters, 278(1), 73–81.
Taylor, D. D., & Gercel-Taylor, C. (2005). Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. British Journal of Cancer, 92(2), 305–311.
Cho, J. A., Park, H., Lim, E. H., Kim, K. H., Choi, J. S., Lee, J. H., et al. (2011). Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecologic Oncology, 123(2), 379–386.
Liang, B., Peng, P., Chen, S., Li, L., Zhang, M., Cao, D., et al. (2013). Characterization and proteomic analysis of ovarian cancer-derived exosomes. Journal of Proteomics, 80C, 171–182.
Taylor, D. D., & Gercel-Taylor, C. (2008). MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecologic Oncology, 110(1), 13–21.
Escrevente, C., Keller, S., Altevogt, P., & Costa, J. (2011). Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer, 11, 108.
Yin, J., Yan, X., Yao, X., Zhang, Y., Shan, Y., Mao, N., et al. (2012). Secretion of annexin A3 from ovarian cancer cells and its association with platinum resistance in ovarian cancer patients. Journal of Cellular and Molecular Medicine, 16(2), 337–348.
Elmasri, W. M., Casagrande, G., Hoskins, E., Kimm, D., & Kohn, E. C. (2009). Cell adhesion in ovarian cancer. Cancer Treatment and Research, 149, 297–318.
Sodek, K. L., Ringuette, M. J., & Brown, T. J. (2007). MT1-MMP is the critical determinant of matrix degradation and invasion by ovarian cancer cells. British Journal of Cancer, 97(3), 358–367.
Davidson, B., Goldberg, I., Gotlieb, W. H., Kopolovic, J., Ben Baruch, G., Nesland, J. M., et al. (1999). High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate with poor survival in ovarian carcinoma. Clinical and Experimental Metastasis, 17(10), 799–808.
Davidson, B., Goldberg, I., Gotlieb, W. H., Kopolovic, J., Ben Baruch, G., Nesland, J. M., et al. (2002). The prognostic value of metalloproteinases and angiogenic factors in ovarian carcinoma. Molecular and Cellular Endocrinology, 187(1–2), 39–45.
Kamat, A. A., Fletcher, M., Gruman, L. M., Mueller, P., Lopez, A., Landen, C. N., Jr., et al. (2006). The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clinical Cancer Research, 12(6), 1707–1714.
Podhajcer, O. L., Benedetti, L. G., Girotti, M. R., Prada, F., Salvatierra, E., & Llera, A. S. (2008). The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Reviews, 27(4), 691–705.
Mok, S. C., Chan, W. Y., Wong, K. K., Muto, M. G., & Berkowitz, R. S. (1996). SPARC, an extracellular matrix protein with tumor-suppressing activity in human ovarian epithelial cells. Oncogene, 12(9), 1895–1901.
Yiu, G. K., Chan, W. Y., Ng, S. W., Chan, P. S., Cheung, K. K., Berkowitz, R. S., et al. (2001). SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. The American Journal of Pathology, 159(2), 609–622.
Said, N. A., Najwer, I., Socha, M. J., Fulton, D. J., Mok, S. C., & Motamed, K. (2007). SPARC inhibits LPA-mediated mesothelial-ovarian cancer cell crosstalk. Neoplasia, 9(1), 23–35.
Said, N., & Motamed, K. (2005). Absence of host-secreted protein acidic and rich in cysteine (SPARC) augments peritoneal ovarian carcinomatosis. The American Journal of Pathology, 167(6), 1739–1752.
Said, N., Socha, M. J., Olearczyk, J. J., Elmarakby, A. A., Imig, J. D., & Motamed, K. (2007). Normalization of the ovarian cancer microenvironment by SPARC. Molecular Cancer Research, 5(10), 1015–1030.
Chang, M. C., Chen, C. A., Chen, P. J., Chiang, Y. C., Chen, Y. L., Mao, T. L., et al. (2012). Mesothelin enhances invasion of ovarian cancer by inducing MMP-7 through MAPK/ERK and JNK pathways. The Biochemical Journal, 442(2), 293–302.
Anttila, M. A., Tammi, R. H., Tammi, M. I., Syrjanen, K. J., Saarikoski, S. V., & Kosma, V. M. (2000). High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Research, 60(1), 150–155.
Voutilainen, K., Anttila, M., Sillanpaa, S., Tammi, R., Tammi, M., Saarikoski, S., et al. (2003). Versican in epithelial ovarian cancer: relation to hyaluronan, clinicopathologic factors and prognosis. International Journal of Cancer, 107(3), 359–364.
Ween, M. P., Oehler, M. K., & Ricciardelli, C. (2011). Role of versican, hyaluronan and CD44 in ovarian cancer metastasis. International Journal of Molecular Sciences, 12(2), 1009–1029.
Fogel, M., Gutwein, P., Mechtersheimer, S., Riedle, S., Stoeck, A., Smirnov, A., et al. (2003). L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet, 362(9387), 869–875.
Stoeck, A., Gast, D., Sanderson, M. P., Issa, Y., Gutwein, P., & Altevogt, P. (2007). L1-CAM in a membrane-bound or soluble form augments protection from apoptosis in ovarian carcinoma cells. Gynecologic Oncology, 104(2), 461–469.
Davidson, B., Goldberg, I., Gotlieb, W. H., Kopolovic, J., Risberg, B., Ben-Baruch, G., et al. (2003). Coordinated expression of integrin subunits, matrix metalloproteinases (MMP), angiogenic genes and Ets transcription factors in advanced-stage ovarian carcinoma: a possible activation pathway? Cancer Metastasis Reviews, 22(1), 103–115.
Shibata, K., Kikkawa, F., Nawa, A., Suganuma, N., & Hamaguchi, M. (1997). Fibronectin secretion from human peritoneal tissue induces Mr 92,000 type IV collagenase expression and invasion in ovarian cancer cell lines. Cancer Research, 57(23), 5416–5420.
Hapke, S., Kessler, H., Luber, B., Benge, A., Hutzler, P., Hofler, H., et al. (2003). Ovarian cancer cell proliferation and motility is induced by engagement of integrin alpha(v)beta3/Vitronectin interaction. Biological Chemistry, 384(7), 1073–1083.
Folkman, J., Watson, K., Ingber, D., & Hanahan, D. (1989). Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature, 339(6219), 58–61.
Carmeliet, P., & Jain, R. K. (2000). Angiogenesis in cancer and other diseases. Nature, 407(6801), 249–257.
Schmid, M. C., & Varner, J. A. (2010). Myeloid cells in the tumor microenvironment: modulation of tumor angiogenesis and tumor inflammation. Journal of Oncology, 2010, 201026.
Su, Y., Zheng, L., Wang, Q., Li, W., Cai, Z., Xiong, S., et al. (2010). Quantity and clinical relevance of circulating endothelial progenitor cells in human ovarian cancer. Journal of Experimental & Clinical Cancer Research, 29, 27.
Peichev, M., Naiyer, A. J., Pereira, D., Zhu, Z., Lane, W. J., Williams, M., et al. (2000). Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood, 95(3), 952–958.
Collino, F., Revelli, A., Massobrio, M., Katsaros, D., Schmitt-Ney, M., Camussi, G., et al. (2009). Epithelial–mesenchymal transition of ovarian tumor cells induces an angiogenic monocyte cell population. Experimental Cell Research, 315(17), 2982–2994.
Alvero, A. B., Fu, H. H., Holmberg, J., Visintin, I., Mor, L., Marquina, C. C., et al. (2009). Stem-like ovarian cancer cells can serve as tumor vascular progenitors. Stem Cells, 27(10), 2405–2413.
Kusumbe, A. P., Mali, A. M., & Bapat, S. A. (2009). CD133-expressing stem cells associated with ovarian metastases establish an endothelial hierarchy and contribute to tumor vasculature. Stem Cells, 27(3), 498–508.
Liby, T. A., Spyropoulos, P., Buff, L. H., Eldridge, J., Beeson, C., Hsu, T., et al. (2012). Akt3 controls vascular endothelial growth factor secretion and angiogenesis in ovarian cancer cells. International Journal of Cancer, 130(3), 532–543.
Su, Y., Zheng, L., Wang, Q., Bao, J., Cai, Z., & Liu, A. (2010). The PI3K/Akt pathway upregulates Id1 and integrin alpha4 to enhance recruitment of human ovarian cancer endothelial progenitor cells. BMC Cancer, 10, 459.
Keyes, K. A., Mann, L., Teicher, B., & Alvarez, E. (2003). Site-dependent angiogenic cytokine production in human tumor xenografts. Cytokine, 21(2), 98–104.
Li, L., Wang, L., Zhang, W., Tang, B., Zhang, J., Song, H., et al. (2004). Correlation of serum VEGF levels with clinical stage, therapy efficacy, tumor metastasis and patient survival in ovarian cancer. Anticancer Research, 24(3b), 1973–1979.
Nascimento, I., Schaer, R., Lemaire, D., Freire, S., Paule, B., Carvalho, S., et al. (2004). Vascular endothelial growth factor (VEGF) levels as a tool to discriminate between malignant and nonmalignant ascites. APMIS, 112(9), 585–587.
Spannuth, W. A., Nick, A. M., Jennings, N. B., Armaiz-Pena, G. N., Mangala, L. S., Danes, C. G., et al. (2009). Functional significance of VEGFR-2 on ovarian cancer cells. International Journal of Cancer, 124(5), 1045–1053.
Ghosh, S., Albitar, L., LeBaron, R., Welch, W. R., Samimi, G., Birrer, M. J., et al. (2010). Up-regulation of stromal versican expression in advanced stage serous ovarian cancer. Gynecologic Oncology, 119(1), 114–120.
Lin, Y. G., Han, L. Y., Kamat, A. A., Merritt, W. M., Landen, C. N., Deavers, M. T., et al. (2007). EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer, 109(2), 332–340.
Hagemann, T., Robinson, S. C., Thompson, R. G., Charles, K., Kulbe, H., & Balkwill, F. R. (2007). Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Molecular Cancer Therapeutics, 6(7), 1993–2002.
Yabushita, H., Shimazu, M., Noguchi, M., Kishida, T., Narumiya, H., Sawaguchi, K., et al. (2003). Vascular endothelial growth factor activating matrix metalloproteinase in ascitic fluid during peritoneal dissemination of ovarian cancer. Oncology Reports, 10(1), 89–95.
Belotti, D., Calcagno, C., Garofalo, A., Caronia, D., Riccardi, E., Giavazzi, R., et al. (2008). Vascular endothelial growth factor stimulates organ-specific host matrix metalloproteinase-9 expression and ovarian cancer invasion. Molecular Cancer Research, 6(4), 525–534.
Sood, A. K., Seftor, E. A., Fletcher, M. S., Gardner, L. M., Heidger, P. M., Buller, R. E., et al. (2001). Molecular determinants of ovarian cancer plasticity. The American Journal of Pathology, 158(4), 1279–1288.
Wang, L., Madigan, M. C., Chen, H., Liu, F., Patterson, K. I., Beretov, J., et al. (2009). Expression of urokinase plasminogen activator and its receptor in advanced epithelial ovarian cancer patients. Gynecologic Oncology, 114(2), 265–272.
Agarwal, A., Tressel, S. L., Kaimal, R., Balla, M., Lam, F. H., Covic, L., et al. (2010). Identification of a metalloprotease-chemokine signaling system in the ovarian cancer microenvironment: implications for antiangiogenic therapy. Cancer Research, 70(14), 5880–5890.
Sonoda, T., Kobayashi, H., Kaku, T., Hirakawa, T., & Nakano, H. (2003). Expression of angiogenesis factors in monolayer culture, multicellular spheroid and in vivo transplanted tumor by human ovarian cancer cell lines. Cancer Letters, 196(2), 229–237.
Zhang, Y., Tang, H., Cai, J., Zhang, T., Guo, J., Feng, D., et al. (2011). Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Letters, 303(1), 47–55.
Castells, M., Thibault, B., Mery, E., Golzio, M., Pasquet, M., Hennebelle, I., et al. (2012). Ovarian ascites-derived Hospicells promote angiogenesis via activation of macrophages. Cancer Letters, 326(1), 59–68.
Jeon, E. S., Heo, S. C., Lee, I. H., Choi, Y. J., Park, J. H., Choi, K. U., et al. (2010). Ovarian cancer-derived lysophosphatidic acid stimulates secretion of VEGF and stromal cell-derived factor-1 alpha from human mesenchymal stem cells. Experimental and Molecular Medicine, 42(4), 280–293.
Schmitt, J., & Matei, D. (2012). Targeting angiogenesis in ovarian cancer. Cancer Treatment Reviews, 38(4), 272–283.
Burger, R. A., Brady, M. F., Bookman, M. A., Fleming, G. F., Monk, B. J., Huang, H., et al. (2011). Incorporation of bevacizumab in the primary treatment of ovarian cancer. The New England Journal of Medicine, 365(26), 2473–2483.
Perren, T. J., Swart, A. M., Pfisterer, J., Ledermann, J. A., Pujade-Lauraine, E., Kristensen, G., et al. (2011). A phase 3 trial of bevacizumab in ovarian cancer. The New England Journal of Medicine, 365(26), 2484–2496.
Yang, G., Rosen, D. G., Zhang, Z., Bast, R. C., Jr., Mills, G. B., Colacino, J. A., et al. (2006). The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America, 103(44), 16472–16477.
Lawrenson, K., Grun, B., Benjamin, E., Jacobs, I. J., Dafou, D., & Gayther, S. A. (2010). Senescent fibroblasts promote neoplastic transformation of partially transformed ovarian epithelial cells in a three-dimensional model of early stage ovarian cancer. Neoplasia, 12(4), 317–325.
Noskova, V., Ahmadi, S., Asander, E., & Casslen, B. (2009). Ovarian cancer cells stimulate uPA gene expression in fibroblastic stromal cells via multiple paracrine and autocrine mechanisms. Gynecologic Oncology, 115(1), 121–126.
Westerlund, A., Hujanen, E., Puistola, U., & Turpeenniemi-Hujanen, T. (1997). Fibroblasts stimulate human ovarian cancer cell invasion and expression of 72-kDa gelatinase A (MMP-2). Gynecologic Oncology, 67(1), 76–82.
Boyd, R. S., & Balkwill, F. R. (1999). MMP-2 release and activation in ovarian carcinoma: the role of fibroblasts. British Journal of Cancer, 80(3–4), 315–321.
Kenny, H. A., Krausz, T., Yamada, S. D., & Lengyel, E. (2007). Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. International Journal of Cancer, 121(7), 1463–1472.
Cai, J., Tang, H., Xu, L., Wang, X., Yang, C., Ruan, S., et al. (2012). Fibroblasts in omentum activated by tumor cells promote ovarian cancer growth, adhesion and invasiveness. Carcinogenesis, 33(1), 20–29.
Xing, F., Saidou, J., & Watabe, K. (2010). Cancer associated fibroblasts (CAFs) in tumor microenvironment. Frontiers in Bioscience, 15, 166–179.
Yao, Q., Qu, X., Yang, Q., Wei, M., & Kong, B. (2009). CLIC4 mediates TGF-beta1-induced fibroblast-to-myofibroblast transdifferentiation in ovarian cancer. Oncology Reports, 22(3), 541–548.
Lai, D., Ma, L., & Wang, F. (2012). Fibroblast activation protein regulates tumor-associated fibroblasts and epithelial ovarian cancer cells. International Journal of Oncology, 41(2), 541–550.
Cannistra, S. A. (2004). Cancer of the ovary. The New England Journal of Medicine, 351(24), 2519–2529.
Niedbala, M. J., Crickard, K., & Bernacki, R. J. (1985). Interactions of human ovarian tumor cells with human mesothelial cells grown on extracellular matrix. An in vitro model system for studying tumor cell adhesion and invasion. Experimental Cell Research, 160(2), 499–513.
Lessan, K., Aguiar, D. J., Oegema, T., Siebenson, L., & Skubitz, A. P. (1999). CD44 and beta1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. The American Journal of Pathology, 154(5), 1525–1537.
Cannistra, S. A., Kansas, G. S., Niloff, J., DeFranzo, B., Kim, Y., & Ottensmeier, C. (1993). Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Research, 53(16), 3830–3838.
Strobel, T., Swanson, L., & Cannistra, S. A. (1997). In vivo inhibition of CD44 limits intra-abdominal spread of a human ovarian cancer xenograft in nude mice: a novel role for CD44 in the process of peritoneal implantation. Cancer Research, 57(7), 1228–1232.
Yeo, T. K., Nagy, J. A., Yeo, K. T., Dvorak, H. F., & Toole, B. P. (1996). Increased hyaluronan at sites of attachment to mesentery by CD44-positive mouse ovarian and breast tumor cells. The American Journal of Pathology, 148(6), 1733–1740.
Burleson, K. M., Casey, R. C., Skubitz, K. M., Pambuccian, S. E., Oegema, T. R., Jr., & Skubitz, A. P. (2004). Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecologic Oncology, 93(1), 170–181.
Offner, F. A., Obrist, P., Stadlmann, S., Feichtinger, H., Klingler, P., Herold, M., et al. (1995). IL-6 secretion by human peritoneal mesothelial and ovarian cancer cells. Cytokine, 7(6), 542–547.
Cronauer, M. V., Stadlmann, S., Klocker, H., Abendstein, B., Eder, I. E., Rogatsch, H., et al. (1999). Basic fibroblast growth factor synthesis by human peritoneal mesothelial cells: induction by interleukin-1. The American Journal of Pathology, 155(6), 1977–1984.
Stadlmann, S., Amberger, A., Pollheimer, J., Gastl, G., Offner, F. A., Margreiter, R., et al. (2005). Ovarian carcinoma cells and IL-1beta-activated human peritoneal mesothelial cells are possible sources of vascular endothelial growth factor in inflammatory and malignant peritoneal effusions. Gynecologic Oncology, 97(3), 784–789.
Ren, J., Xiao, Y. J., Singh, L. S., Zhao, X., Zhao, Z., Feng, L., et al. (2006). Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Research, 66(6), 3006–3014.
Uccelli, A., Moretta, L., & Pistoia, V. (2008). Mesenchymal stem cells in health and disease. Nature Reviews Immunology, 8(9), 726–736.
Jiang, J., Chen, W., Zhuang, R., Song, T., & Li, P. (2010). The effect of endostatin mediated by human mesenchymal stem cells on ovarian cancer cells in vitro. Journal of Cancer Research and Clinical Oncology, 136(6), 873–881.
Jeon, E. S., Moon, H. J., Lee, M. J., Song, H. Y., Kim, Y. M., Cho, M., et al. (2008). Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblast-like cells. Stem Cells, 26(3), 789–797.
Lis, R., Touboul, C., Mirshahi, P., Ali, F., Mathew, S., Nolan, D. J., et al (2010). Tumor associated mesenchymal stem cells protects ovarian cancer cells from hyperthermia through CXCL12. Int J Cancer
McLean, K., Gong, Y., Choi, Y., Deng, N., Yang, K., Bai, S., et al. (2011). Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. The Journal of Clinical Investigation, 121(8), 3206–3219.
Rafii, A., Mirshahi, P., Poupot, M., Faussat, A. M., Simon, A., Ducros, E., et al. (2008). Oncologic trogocytosis of an original stromal cells induces chemoresistance of ovarian tumours. PLoS One, 3(12), e3894.
Pasquet, M., Golzio, M., Mery, E., Rafii, A., Benabbou, N., Mirshahi, P., et al. (2010). Hospicells (ascites-derived stromal cells) promote tumorigenicity and angiogenesis. International Journal of Cancer, 126(9), 2090–2101.
Castells, M., Thibault, B., Mery, E., Golzio, M., Pasquet, M., Hennebelle, I., et al (2012). Ovarian ascites-derived Hospicells promote angiogenesis via activation of macrophages. Cancer Lett
Martinet, L., Poupot, R., Mirshahi, P., Rafii, A., Fournie, J. J., Mirshahi, M., et al. (2010). Hospicells derived from ovarian cancer stroma inhibit T-cell immune responses. International Journal of Cancer, 126(9), 2143–2152.
Nieman, K. M., Kenny, H. A., Penicka, C. V., Ladanyi, A., Buell-Gutbrod, R., Zillhardt, M. R., et al. (2011). Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature Medicine, 17(11), 1498–1503.
Zhang, Y., Daquinag, A. C., Amaya-Manzanares, F., Sirin, O., Tseng, C., & Kolonin, M. G. (2012). Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Research, 72(20), 5198–5208.
Altintas, M. M., Azad, A., Nayer, B., Contreras, G., Zaias, J., Faul, C., et al. (2011). Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. Journal of Lipid Research, 52(3), 480–488.
Subbaramaiah, K., Howe, L. R., Bhardwaj, P., Du, B., Gravaghi, C., Yantiss, R. K., et al. (2011). Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prevention Research (Philadelphia, Pa.), 4(3), 329–346.
Roodhart, J. M., Daenen, L. G., Stigter, E. C., Prins, H. J., Gerrits, J., Houthuijzen, J. M., et al. (2011). Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell, 20(3), 370–383.
Ko, S. Y., Barengo, N., Ladanyi, A., Lee, J. S., Marini, F., Lengyel, E., et al. (2012). HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. The Journal of Clinical Investigation, 122(10), 3603–3617.
Kidd, S., Spaeth, E., Watson, K., Burks, J., Lu, H., Klopp, A., et al. (2012). Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One, 7(2), e30563.
Protani, M., Coory, M., & Martin, J. H. (2010). Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Research and Treatment, 123(3), 627–635.
Kaaks, R., Lukanova, A., & Kurzer, M. S. (2002). Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiology, Biomarkers and Prevention, 11(12), 1531–1543.
Fotopoulou, C., Richter, R., Braicu, E. I., Kuhberg, M., Feldheiser, A., Schefold, J. C., et al. (2011). Impact of obesity on operative morbidity and clinical outcome in primary epithelial ovarian cancer after optimal primary tumor debulking. Annals of Surgical Oncology, 18(9), 2629–2637.
Reeves, G. K., Pirie, K., Beral, V., Green, J., Spencer, E., & Bull, D. (2007). Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ, 335(7630), 1134.
Protani, M. M., Nagle, C. M., & Webb, P. M. (2012). Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prevention Research (Philadelphia, Pa.), 5(7), 901–910.
Olsen, C. M., Nagle, C. M., Whiteman, D. C., Purdie, D. M., Green, A. C., & Webb, P. M. (2008). Body size and risk of epithelial ovarian and related cancers: a population-based case-control study. International Journal of Cancer, 123(2), 450–456.
Olsen, C. M., Nagle, C. M., Whiteman, D. C., Ness, R., Pearce, C. L., Pike, M. C., et al. (2013). Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocrine-Related Cancer, 20(2), 251–262.
Zhang, L., Conejo-Garcia, J. R., Katsaros, D., Gimotty, P. A., Massobrio, M., Regnani, G., et al. (2003). Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England Journal of Medicine, 348(3), 203–213.
Fialova, A., Partlova, S., Sojka, L., Hromadkova, H., Brtnicky, T., Fucikova, J., et al (2012). Dynamics of T-cell infiltration during the course of ovarian cancer: the gradual shift from a Th17 effector cell response to a predominant infiltration by regulatory T-cells. Int J Cancer
Milne, K., Alexander, C., Webb, J. R., Sun, W., Dillon, K., Kalloger, S. E., et al. (2012). Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. Journal of Translational Medicine, 10, 33.
Wei, S., Kryczek, I., Zou, L., Daniel, B., Cheng, P., Mottram, P., et al. (2005). Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Research, 65(12), 5020–5026.
Curiel, T. J., Cheng, P., Mottram, P., Alvarez, X., Moons, L., Evdemon-Hogan, M., et al. (2004). Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Research, 64(16), 5535–5538.
Huarte, E., Cubillos-Ruiz, J. R., Nesbeth, Y. C., Scarlett, U. K., Martinez, D. G., Buckanovich, R. J., et al. (2008). Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Research, 68(18), 7684–7691.
Labidi-Galy, S. I., Sisirak, V., Meeus, P., Gobert, M., Treilleux, I., Bajard, A., et al. (2011). Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Research, 71(16), 5423–5434.
Labidi-Galy, S. I., Treilleux, I., Goddard-Leon, S., Combes, J. D., Blay, J. Y., Ray-Coquard, I., et al. (2012). Plasmacytoid dendritic cells infiltrating ovarian cancer are associated with poor prognosis. Oncoimmunology, 1(3), 380–382.
Sato, E., Olson, S. H., Ahn, J., Bundy, B., Nishikawa, H., Qian, F., et al. (2005). Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America, 102(51), 18538–18543.
Hamanishi, J., Mandai, M., Iwasaki, M., Okazaki, T., Tanaka, Y., Yamaguchi, K., et al. (2007). Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America, 104(9), 3360–3365.
Lai, D., Wang, F., Chen, Y., Wang, C., Liu, S., Lu, B., et al. (2011). Human ovarian cancer stem-like cells can be efficiently killed by gammadelta T lymphocytes. Immunother: Cancer Immunol.
Peng, D. J., Liu, R., & Zou, W. (2012). Regulatory T cells in human ovarian cancer. Journal of Oncology, 2012, 345164.
Alvero, A. B., Montagna, M. K., Craveiro, V., Liu, L., & Mor, G. (2011). Distinct subpopulations of epithelial ovarian cancer cells can differentially induce macrophages and T regulatory cells toward a pro-tumor phenotype. Immunol: Am J Reprod.
Curiel, T. J., Coukos, G., Zou, L., Alvarez, X., Cheng, P., Mottram, P., et al. (2004). Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Medicine, 10(9), 942–949.
DeNardo, D. G., Barreto, J. B., Andreu, P., Vasquez, L., Tawfik, D., Kolhatkar, N., et al. (2009). CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell, 16(2), 91–102.
Gavalas, N. G., Karadimou, A., Dimopoulos, M. A., & Bamias, A. (2010). Immune response in ovarian cancer: how is the immune system involved in prognosis and therapy: potential for treatment utilization. Clinical and Developmental Immunology, 2010, 791603.
Bettelli, E., Korn, T., Oukka, M., & Kuchroo, V. K. (2008). Induction and effector functions of T(H)17 cells. Nature, 453(7198), 1051–1057.
Miyahara, Y., Odunsi, K., Chen, W., Peng, G., Matsuzaki, J., & Wang, R. F. (2008). Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America, 105(40), 15505–15510.
Leveque, L., Deknuydt, F., Bioley, G., Old, L. J., Matsuzaki, J., Odunsi, K., et al. (2009). Interleukin 2-mediated conversion of ovarian cancer-associated CD4+ regulatory T cells into proinflammatory interleukin 17-producing helper T cells. Journal of Immunotherapy, 32(2), 101–108.
Lan, C., Huang, X., Lin, S., Huang, H., Cai, Q., Lu, J., et al (2013). High density of IL-17-producing cells is associated with improved prognosis for advanced epithelial ovarian cancer. Cell Tissue Res
Biswas, S. K., Sica, A., & Lewis, C. E. (2008). Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. Journal of Immunology, 180(4), 2011–2017.
Hagemann, T., Wilson, J., Burke, F., Kulbe, H., Li, N. F., Pluddemann, A., et al. (2006). Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. Journal of Immunology, 176(8), 5023–5032.
Robinson-Smith, T. M., Isaacsohn, I., Mercer, C. A., Zhou, M., Van, R. N., Husseinzadeh, N., et al. (2007). Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Research, 67(12), 5708–5716.
Duluc, D., Delneste, Y., Tan, F., Moles, M. P., Grimaud, L., Lenoir, J., et al. (2007). Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood, 110(13), 4319–4330.
Kryczek, I., Zou, L., Rodriguez, P., Zhu, G., Wei, S., Mottram, P., et al. (2006). B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. The Journal of Experimental Medicine, 203(4), 871–881.
Kryczek, I., Wei, S., Zhu, G., Myers, L., Mottram, P., Cheng, P., et al. (2007). Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Research, 67(18), 8900–8905.
Fridlender, Z. G., & Albelda, S. M. (2012). Tumor-associated neutrophils: friend or foe? Carcinogenesis, 33(5), 949–955.
Piccard, H., Muschel, R. J., & Opdenakker, G. (2012). On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Critical Reviews in Oncology/Hematology, 82(3), 296–309.
Gonzalez, M. A., Bratos, R., Marquez, R., Alonso, S., & Chiva, L. (2013). Bevacizumab as front-line treatment for newly diagnosed epithelial cancer. Expert Review of Anticancer Therapy, 13(2), 123–129.
Banerjee, S., & Kaye, S. B. (2013). New strategies in the treatment of ovarian cancer—current clinical perspectives and future potential. Cancer Res: Clin.
Acknowledgments
The manuscript was revised by AngloScribe, Nîmes for English language editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thibault, B., Castells, M., Delord, JP. et al. Ovarian cancer microenvironment: implications for cancer dissemination and chemoresistance acquisition. Cancer Metastasis Rev 33, 17–39 (2014). https://doi.org/10.1007/s10555-013-9456-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-013-9456-2