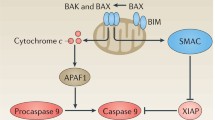
Abstract
The alteration in expression of B cell lymphoma-2 (Bcl-2) family of protein members in cancer is involved mainly in the regulation of apoptosis. Bcl-2 family proteins are currently used as major targets in the development of methods to improve treatment outcomes for cancer patients that underwent clinical trials. Although many agents have been developed for targeting Bcl-2 in the past decade, some previous attempts to target Bcl-2 have not resulted in beneficial clinical outcome for reasons unknown. Here, we propose that this was due in part for not considering the cellular level of a different antiapoptotic protein, i.e., galectin-3 (Gal-3). Gal-3 is a member of the β-galactoside binding protein family and a multifunctional oncogenic protein which regulates cell growth, cell adhesion, cell proliferation, angiogenesis, and apoptosis. Gal-3 is the sole protein that contains the NWGR anti-death motif of the Bcl-2 family and inhibits cell apoptosis induced by chemotherapeutic agents through phosphorylation, translocation and regulation of survival signaling pathways. It is now established that Gal-3 is a candidate target protein to suppress antiapoptotic activity and anticancer drug resistance. In this review, we describe the role and relevance of Gal-3 and Bcl-2 protein family in the regulation of apoptosis and propose a novel combination therapy modality. Combination therapy that targets Gal-3 could be essential for improvement of the efficacy of Bcl-2 targeting therapy in cancers and should be studied in future clinical trials. Otherwise, not considering Gal-3 cellular level could lead to trial failure.



Similar content being viewed by others
References
Danial, N. N., & Korsmeyer, S. J. (2004). Cell death: critical control points. Cell, 116, 205–219.
Cotter, T. G. (2009). Apoptosis and cancer: the genesis of a research field. Nature reviews. Cancer, 9, 501–507.
Green, D. R., & Reed, J. C. (1998). Mitochondria and apoptosis. Science, 281, 1309–1312.
Haldar, S., Basu, A., & Croce, C. M. (1998). Serine-70 is one of the critical sites for drug-induced Bcl2 phosphorylation in cancer cells. Cancer Research, 58, 1609–1615.
Ito, T., Deng, X., Carr, B., & May, W. S. (1997). Bcl-2 phosphorylation required for anti-apoptosis function. The Journal of Biological Chemistry, 272, 11671–11673.
Ojala, P. M., Yamamoto, K., Castanos-Velez, E., Biberfeld, P., Korsmeyer, S. J., & Makela, T. P. (2000). The apoptotic v-cyclin-CDK6 complex phosphorylates and inactivates Bcl-2. Nature Cell Biology, 2, 819–825.
Pratesi, G., Perego, P., & Zunino, F. (2001). Role of Bcl-2 and its post-transcriptional modification in response to antitumor therapy. Biochemical Pharmacology, 61, 381–386.
Ruvolo, P. P., Deng, X., Carr, B. K., & May, W. S. (1998). A functional role for mitochondrial protein kinase Calpha in Bcl2 phosphorylation and suppression of apoptosis. The Journal of Biological Chemistry, 273, 25436–25442.
Salah-Eldin, A. E., Inoue, S., Tsukamoto, S., Aoi, H., & Tsuda, M. (2003). An association of Bcl-2 phosphorylation and Bax localization with their functions after hyperthermia and paclitaxel treatment. International journal of cancer. Journal International du Cancer, 103, 53–60.
Yamamoto, K., Ichijo, H., & Korsmeyer, S. J. (1999). BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Molecular and Cellular Biology, 19, 8469–8478.
Yokote, H., Terada, T., Matsumoto, H., Kakishita, K., Kinoshita, Y., Nakao, N., et al. (2000). Dephosphorylation-induced decrease of anti-apoptotic function of Bcl-2 in neuronally differentiated P19 cells following ischemic insults. Brain Research, 857, 78–86.
Leber, B., Geng, F., Kale, J., & Andrews, D. W. (2010). Drugs targeting Bcl-2 family members as an emerging strategy in cancer. Expert Reviews in Molecular Medicine, 12, e28.
Roberts, A. W., Seymour, J. F., Brown, J. R., Wierda, W. G., Kipps, T. J., Khaw, S. L., et al. (2012). Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 30, 488–496.
Barondes, S. H., Cooper, D. N., Gitt, M. A., & Leffler, H. (1994). Galectins. Structure and function of a large family of animal lectins. The Journal of Biological Chemistry, 269, 20807–20810.
Ochieng, J., Fridman, R., Nangia-Makker, P., Kleiner, D. E., Liotta, L. A., Stetler-Stevenson, W. G., et al. (1994). Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry, 33, 14109–14114.
Liu, F. T., Hsu, D. K., Zuberi, R. I., Kuwabara, I., Chi, E. Y., & Henderson, W. R., Jr. (1995). Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. The American Journal of Pathology, 147, 1016–1028.
Xu, X. C., el-Naggar, A. K., & Lotan, R. (1995). Differential expression of galectin-1 and galectin-3 in thyroid tumors. Potential diagnostic implications. The American journal of Pathology, 147, 815–822.
Hsu, D. K., Hammes, S. R., Kuwabara, I., Greene, W. C., & Liu, F. T. (1996). Human T lymphotropic virus-I infection of human T lymphocytes induces expression of the beta-galactoside-binding lectin, galectin-3. The American Journal of Pathology, 148, 1661–1670.
Konstantinov, K. N., Robbins, B. A., & Liu, F. T. (1996). Galectin-3, a beta-galactoside-binding animal lectin, is a marker of anaplastic large-cell lymphoma. The American Journal of Pathology, 148, 25–30.
van den Brule, F. A., Buicu, C., Berchuck, A., Bast, R. C., Deprez, M., Liu, F. T., et al. (1996). Expression of the 67-kD laminin receptor, galectin-1, and galectin-3 in advanced human uterine adenocarcinoma. Human Pathology, 27, 1185–1191.
Lotan, R., Ito, H., Yasui, W., Yokozaki, H., Lotan, D., & Tahara, E. (1994). Expression of a 31-kDa lactoside-binding lectin in normal human gastric mucosa and in primary and metastatic gastric carcinomas. International journal of cancer. Journal International du Cancer, 56, 474–480.
Schoeppner, H. L., Raz, A., Ho, S. B., & Bresalier, R. S. (1995). Expression of an endogenous galactose-binding lectin correlates with neoplastic progression in the colon. Cancer, 75, 2818–2826.
Barondes, S. H., Castronovo, V., Cooper, D. N., Cummings, R. D., Drickamer, K., Feizi, T., et al. (1994). Galectins: a family of animal beta-galactoside-binding lectins. Cell, 76, 597–598.
Perillo, N. L., Marcus, M. E., & Baum, L. G. (1998). Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. Journal of Molecular Medicine, 76, 402–412.
Inohara, H., Akahani, S., & Raz, A. (1998). Galectin-3 stimulates cell proliferation. Experimental Cell Research, 245, 294–302.
Inohara, H., & Raz, A. (1995). Functional evidence that cell surface galectin-3 mediates homotypic cell adhesion. Cancer Research, 55, 3267–3271.
Nangia-Makker, P., Honjo, Y., Sarvis, R., Akahani, S., Hogan, V., Pienta, K. J., et al. (2000). Galectin-3 induces endothelial cell morphogenesis and angiogenesis. The American journal of Pathology, 156, 899–909.
Yang, R. Y., & Liu, F. T. (2003). Galectins in cell growth and apoptosis. Cellular and Molecular Life Sciences: CMLS, 60, 267–276.
Fukumori, T., Takenaka, Y., Oka, N., Yoshii, T., Hogan, V., Inohara, H., et al. (2004). Endogenous galectin-3 determines the routing of CD95 apoptotic signaling pathways. Cancer Research, 64, 3376–3379.
Nangia-Makker, P., Nakahara, S., Hogan, V., & Raz, A. (2007). Galectin-3 in apoptosis, a novel therapeutic target. Journal of Bioenergetics and Biomembranes, 39, 79–84.
Akahani, S., Nangia-Makker, P., Inohara, H., Kim, H. R., & Raz, A. (1997). Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Research, 57, 5272–5276.
Kim, H. R., Lin, H. M., Biliran, H., & Raz, A. (1999). Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Research, 59, 4148–4154.
Lin, H. M., Moon, B. K., Yu, F., & Kim, H. R. (2000). Galectin-3 mediates genistein-induced G(2)/M arrest and inhibits apoptosis. Carcinogenesis, 21, 1941–1945.
Yoshii, T., Fukumori, T., Honjo, Y., Inohara, H., Kim, H. R., & Raz, A. (2002). Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. The Journal of Biological Chemistry, 277, 6852–6857.
Fukumori, T., Oka, N., Takenaka, Y., Nangia-Makker, P., Elsamman, E., Kasai, T., et al. (2006). Galectin-3 regulates mitochondrial stability and antiapoptotic function in response to anticancer drug in prostate cancer. Cancer Research, 66, 3114–3119.
Takenaka, Y., Fukumori, T., Yoshii, T., Oka, N., Inohara, H., Kim, H. R., et al. (2004). Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Molecular and Cellular Biology, 24, 4395–4406.
Nakahara, S., Hogan, V., Inohara, H., & Raz, A. (2006). Importin-mediated nuclear translocation of galectin-3. The Journal of Biological Chemistry, 281, 39649–39659.
van den Brule, F. A., Waltregny, D., Liu, F. T., & Castronovo, V. (2000). Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. International journal of cancer. Journal International du Cancer, 89, 361–367.
Yu, F., Finley, R. L., Jr., Raz, A., & Kim, H. R. (2002). Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. The Journal of Biological Chemistry, 277, 15819–15827.
Reed, J. C., & Pellecchia, M. (2005). Apoptosis-based therapies for hematologic malignancies. Blood, 106, 408–418.
Youle, R. J., & Strasser, A. (2008). The BCL-2 protein family: opposing activities that mediate cell death. Nature reviews. Molecular Cell Biology, 9, 47–59.
Reed, J. C. (2008). Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood, 111, 3322–3330.
Weyhenmeyer, B., Murphy, A. C., Prehn, J. H., & Murphy, B. M. (2012). Targeting the anti-apoptotic Bcl-2 family members for the treatment of cancer. Experimental Oncology, 34, 192–199.
Kang, M. H., & Reynolds, C. P. (2009). Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 15, 1126–1132.
Wajant, H. (2002). The Fas signaling pathway: more than a paradigm. Science, 296, 1635–1636.
Adams, J. M., & Cory, S. (2007). The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene, 26, 1324–1337.
Kim, H., Rafiuddin-Shah, M., Tu, H. C., Jeffers, J. R., Zambetti, G. P., Hsieh, J. J., et al. (2006). Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nature Cell Biology, 8, 1348–1358.
Letai, A., Bassik, M. C., Walensky, L. D., Sorcinelli, M. D., Weiler, S., & Korsmeyer, S. J. (2002). Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell, 2, 183–192.
Chen, L., Willis, S. N., Wei, A., Smith, B. J., Fletcher, J. I., Hinds, M. G., et al. (2005). Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Molecular Cell, 17, 393–403.
Willis, S. N., Fletcher, J. I., Kaufmann, T., van Delft, M. F., Chen, L., Czabotar, P. E., et al. (2007). Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science, 315, 856–859.
Leber, B., Lin, J., & Andrews, D. W. (2007). Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis: An International Journal on Programmed Cell Death, 12, 897–911.
Yin, X. M., Oltvai, Z. N., & Korsmeyer, S. J. (1994). BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature, 369, 321–323.
Davids, M. S., & Letai, A. (2012). Targeting the B-cell lymphoma/leukemia 2 family in cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 30, 3127–3135.
Klasa, R. J., Gillum, A. M., Klem, R. E., & Frankel, S. R. (2002). Oblimersen Bcl-2 antisense: facilitating apoptosis in anticancer treatment. Antisense & Nucleic Acid Drug Development, 12, 193–213.
Chi, K. N., Gleave, M. E., Klasa, R., Murray, N., Bryce, C., Lopes de Menezes, D. E., et al. (2001). A phase I dose-finding study of combined treatment with an antisense Bcl-2 oligonucleotide (Genasense) and mitoxantrone in patients with metastatic hormone-refractory prostate cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 7, 3920–3927.
Marcucci, G., Byrd, J. C., Dai, G., Klisovic, M. I., Kourlas, P. J., Young, D. C., et al. (2003). Phase 1 and pharmacodynamic studies of G3139, a Bcl-2 antisense oligonucleotide, in combination with chemotherapy in refractory or relapsed acute leukemia. Blood, 101, 425–432.
Tolcher, A. W., Kuhn, J., Schwartz, G., Patnaik, A., Hammond, L. A., Thompson, I., et al. (2004). A Phase I pharmacokinetic and biological correlative study of oblimersen sodium (genasense, g3139), an antisense oligonucleotide to the bcl-2 mRNA, and of docetaxel in patients with hormone-refractory prostate cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 10, 5048–5057.
Bedikian, A. Y., Millward, M., Pehamberger, H., Conry, R., Gore, M., Trefzer, U., et al. (2006). Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 24, 4738–4745.
O’Brien, S., Moore, J. O., Boyd, T. E., Larratt, L. M., Skotnicki, A. B., Koziner, B., et al. (2009). 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 27, 5208–5212.
Rudin, C. M., Salgia, R., Wang, X., Hodgson, L. D., Masters, G. A., Green, M., et al. (2008). Randomized phase II Study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 26, 870–876.
Wei, J., Kitada, S., Rega, M. F., Emdadi, A., Yuan, H., Cellitti, J., et al. (2009). Apogossypol derivatives as antagonists of antiapoptotic Bcl-2 family proteins. Molecular Cancer Therapeutics, 8, 904–913.
Mohammad, R. M., Wang, S., Aboukameel, A., Chen, B., Wu, X., Chen, J., et al. (2005). Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-X(L) [(-)-gossypol] against diffuse large cell lymphoma. Molecular Cancer Therapeutics, 4, 13–21.
Shelley, M. D., Hartley, L., Groundwater, P. W., & Fish, R. G. (2000). Structure-activity studies on gossypol in tumor cell lines. Anti-Cancer Drugs, 11, 209–216.
Vogler, M., Weber, K., Dinsdale, D., Schmitz, I., Schulze-Osthoff, K., Dyer, M. J., et al. (2009). Different forms of cell death induced by putative BCL2 inhibitors. Cell Death and Differentiation, 16, 1030–1039.
Shore, G. C., & Viallet, J. (2005). Modulating the bcl-2 family of apoptosis suppressors for potential therapeutic benefit in cancer. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. Education Program, 226–230.
Zhai, D., Jin, C., Satterthwait, A. C., & Reed, J. C. (2006). Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death And Differentiation, 13, 1419–1421.
O’Brien, S. M., Claxton, D. F., Crump, M., Faderl, S., Kipps, T., Keating, M. J., et al. (2009). Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood, 113, 299–305.
Schimmer, A. D., O’Brien, S., Kantarjian, H., Brandwein, J., Cheson, B. D., Minden, M. D., et al. (2008). A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 14, 8295–8301.
Oki, Y., Copeland, A., Hagemeister, F., Fayad, L. E., Fanale, M., Romaguera, J., et al. (2012). Experience with obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist in patients with relapsed or refractory classical Hodgkin lymphoma. Blood, 119, 2171–2172.
Paik, P. K., Rudin, C. M., Pietanza, M. C., Brown, A., Rizvi, N. A., Takebe, N., et al. (2011). A phase II study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in relapsed small cell lung cancer. Lung Cancer, 74, 481–485.
Trudel, S., Li, Z. H., Rauw, J., Tiedemann, R. E., Wen, X. Y., & Stewart, A. K. (2007). Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood, 109, 5430–5438.
Oltersdorf, T., Elmore, S. W., Shoemaker, A. R., Armstrong, R. C., Augeri, D. J., Belli, B. A., et al. (2005). An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature, 435, 677–681.
Kang, M. H., Kang, Y. H., Szymanska, B., Wilczynska-Kalak, U., Sheard, M. A., Harned, T. M., et al. (2007). Activity of vincristine, L-ASP, and dexamethasone against acute lymphoblastic leukemia is enhanced by the BH3-mimetic ABT-737 in vitro and in vivo. Blood, 110, 2057–2066.
Konopleva, M., Contractor, R., Tsao, T., Samudio, I., Ruvolo, P. P., Kitada, S., et al. (2006). Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell, 10, 375–388.
van Delft, M. F., Wei, A. H., Mason, K. D., Vandenberg, C. J., Chen, L., Czabotar, P. E., et al. (2006). The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell, 10, 389–399.
Tse, C., Shoemaker, A. R., Adickes, J., Anderson, M. G., Chen, J., Jin, S., et al. (2008). ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Research, 68, 3421–3428.
Gandhi, L., Camidge, D. R., Ribeiro de Oliveira, M., Bonomi, P., Gandara, D., Khaira, D., et al. (2011). Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 29, 909–916.
Wilson, W. H., O’Connor, O. A., Czuczman, M. S., LaCasce, A. S., Gerecitano, J. F., Leonard, J. P., et al. (2010). Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. The lancet Oncology, 11, 1149–1159.
Rudin, C. M., Hann, C. L., Garon, E. B., Ribeiro de Oliveira, M., Bonomi, P. D., Camidge, D. R., et al. (2012). Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 18, 3163–3169.
Mazumder, S., Choudhary, G. S., Al-Harbi, S., & Almasan, A. (2012). Mcl-1 Phosphorylation defines ABT-737 resistance that can be overcome by increased NOXA expression in leukemic B cells. Cancer Research, 72, 3069–3079.
Xu, H., & Krystal, G. W. (2010). Actinomycin D decreases Mcl-1 expression and acts synergistically with ABT-737 against small cell lung cancer cell lines. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 16, 4392–4400.
Zhang, C., Cai, T. Y., Zhu, H., Yang, L. Q., Jiang, H., Dong, X. W., et al. (2011). Synergistic antitumor activity of gemcitabine and ABT-737 in vitro and in vivo through disrupting the interaction of USP9X and Mcl-1. Molecular Cancer Therapeutics, 10, 1264–1275.
Yang, R. Y., Rabinovich, G. A., & Liu, F. T. (2008). Galectins: structure, function and therapeutic potential. Expert Reviews in Molecular Medicine, 10, e17.
Prieto, V. G., Mourad-Zeidan, A. A., Melnikova, V., Johnson, M. M., Lopez, A., Diwan, A. H., et al. (2006). Galectin-3 expression is associated with tumor progression and pattern of sun exposure in melanoma. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 12, 6709–6715.
Kopper, L., & Timar, J. (2006). Genomics of renal cell cancer—does it provide breakthrough? Pathology Oncology Research: POR, 12, 5–11.
Califice, S., Castronovo, V., & Van Den Brule, F. (2004). Galectin-3 and cancer (Review). International Journal of Oncology, 25, 983–992.
Fukumori, T., Kanayama, H. O., & Raz, A. (2007). The role of galectin-3 in cancer drug resistance. Drug Resistance Updates: Reviews and Commentaries in Antimicrobial and Anticancer Chemotherapy, 10, 101–108.
Huflejt, M. E., Turck, C. W., Lindstedt, R., Barondes, S. H., & Leffler, H. (1993). L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. The Journal of Biological Chemistry, 268, 26712–26718.
Mazurek, N., Conklin, J., Byrd, J. C., Raz, A., & Bresalier, R. S. (2000). Phosphorylation of the beta-galactoside-binding protein galectin-3 modulates binding to its ligands. The Journal of Biological Chemistry, 275, 36311–36315.
Matarrese, P., Fusco, O., Tinari, N., Natoli, C., Liu, F. T., Semeraro, M. L., et al. (2000). Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. International journal of cancer. Journal International du Cancer, 85, 545–554.
Yang, R. Y., Hsu, D. K., & Liu, F. T. (1996). Expression of galectin-3 modulates T-cell growth and apoptosis. Proceedings of the National Academy of Sciences of the United States of America, 93, 6737–6742.
Nakahara, S., Oka, N., Wang, Y., Hogan, V., Inohara, H., & Raz, A. (2006). Characterization of the nuclear import pathways of galectin-3. Cancer Research, 66, 9995–10006.
Oka, N., Nakahara, S., Takenaka, Y., Fukumori, T., Hogan, V., Kanayama, H. O., et al. (2005). Galectin-3 inhibits tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by activating Akt in human bladder carcinoma cells. Cancer Research, 65, 7546–7553.
Pieters, R. J. (2006). Inhibition and detection of galectins. Chembiochem: A European journal of Chemical Biology, 7, 721–728.
Delaine, T., Cumpstey, I., Ingrassia, L., Le Mercier, M., Okechukwu, P., Leffler, H., et al. (2008). Galectin-inhibitory thiodigalactoside ester derivatives have antimigratory effects in cultured lung and prostate cancer cells. Journal of Medicinal Chemistry, 51, 8109–8114.
Lin, C. I., Whang, E. E., Donner, D. B., Jiang, X., Price, B. D., Carothers, A. M., et al. (2009). Galectin-3 targeted therapy with a small molecule inhibitor activates apoptosis and enhances both chemosensitivity and radiosensitivity in papillary thyroid cancer. Molecular Cancer Research: MCR, 7, 1655–1662.
Inohara, H., & Raz, A. (1994). Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconjugate Journal, 11, 527–532.
Nangia-Makker, P., Hogan, V., Honjo, Y., Baccarini, S., Tait, L., Bresalier, R., et al. (2002). Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. Journal of the National Cancer Institute, 94, 1854–1862.
Pienta, K. J., Naik, H., Akhtar, A., Yamazaki, K., Replogle, T. S., Lehr, J., et al. (1995). Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. Journal of the National Cancer Institute, 87, 348–353.
Streetly, M. J., Maharaj, L., Joel, S., Schey, S. A., Gribben, J. G., & Cotter, F. E. (2010). GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death. Blood, 115, 3939–3948.
Chauhan, D., Li, G., Podar, K., Hideshima, T., Neri, P., He, D., et al. (2005). A novel carbohydrate-based therapeutic GCS-100 overcomes bortezomib resistance and enhances dexamethasone-induced apoptosis in multiple myeloma cells. Cancer Research, 65, 8350–8358.
Hanada, M., Aime-Sempe, C., Sato, T., & Reed, J. C. (1995). Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. The Journal of Biological Chemistry, 270, 11962–11969.
Acknowledgments
The work was supported by the National Institute of Health grant R37CA46120 (to A.R.).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harazono, Y., Nakajima, K. & Raz, A. Why anti-Bcl-2 clinical trials fail: a solution. Cancer Metastasis Rev 33, 285–294 (2014). https://doi.org/10.1007/s10555-013-9450-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-013-9450-8