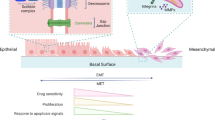
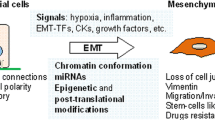
Abstract
The molecular mechanisms underlying cancer progression and metastasis are still poorly understood. In recent years, the epithelial-to-mesenchymal transition (EMT), a traditional phenomenon revealed in embryonic development, has been gradually accepted as a potential mechanism underlying cancer progression and metastasis. Many cell signaling pathways involved in development have been shown to contribute to EMT. An increasing number of genetic and epigenetic elements have been discovered, and their cross-talk relationship in EMT remains to be explored. In addition, accumulating experimental evidence suggests that EMT plays a critical role in different aspects of cancer progression, such as metastasis, stem cell traits, and chemoresistance. However, there are some disagreements and debate about these studies, which raise critical questions worthy of further investigation. Solving these questions will lead to a more complete understanding of cancer metastasis. Due to the close relationship of EMT to cancer metastasis and chemoresistance, targeting EMT or reversing EMT is likely to lead to novel therapeutic approaches for the treatment of human cancers.

Similar content being viewed by others
References
Acloque, H., Thiery, J. P., & Nieto, M. A. (2008). The physiology and pathology of the EMT. EMBO Reports, 9(4), 322–326. doi:10.1038/embor.2008.30.
Baum, B., Settleman, J., & Quinlan, M. P. (2008). Transitions between epithelial and mesenchymal states in development and disease. Seminars in Cell & Developmental Biology, 19(3), 294–308. doi:10.1016/j.semcdb.2008.02.001.
Thiery, J. P. (2002). Epithelial–mesenchymal transitions in tumour progression. Nature Reviews. Cancer, 2(6), 442–454. doi:10.1038/nrc822nrc822.
Thiery, J. P., & Sleeman, J. P. (2006). Complex networks orchestrate epithelial–mesenchymal transitions. Nature Reviews Molecular Cell Biology, 7(2), 131–142. doi:10.1038/nrm1835.
Yang, J., & Weinberg, R. A. (2008). Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Developmental Cell, 14(6), 818–829. doi:10.1016/j.devcel.2008.05.009.
Davies, J. A. (1996). Mesenchyme to epithelium transition during development of the mammalian kidney tubule. Acta Anat (Basel), 156(3), 187–201.
Voulgari, A., & Pintzas, A. (2009). Epithelial–mesenchymal transition in cancer metastasis: Mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochimica et Biophysica Acta, 1796(2), 75–90. doi:10.1016/j.bbcan.2009.03.002.
Barr, S., Thomson, S., Buck, E., Russo, S., Petti, F., Sujka-Kwok, I., et al. (2008). Bypassing cellular EGF receptor dependence through epithelial-to-mesenchymal-like transitions. Clinical & Experimental Metastasis, 25(6), 685–693. doi:10.1007/s10585-007-9121-7.
Huber, M. A., Kraut, N., & Beug, H. (2005). Molecular requirements for epithelial–mesenchymal transition during tumor progression. Current Opinion in Cell Biology, 17(5), 548–558. doi:10.1016/j.ceb.2005.08.001.
Moustakas, A., & Heldin, C. H. (2007). Signaling networks guiding epithelial–mesenchymal transitions during embryogenesis and cancer progression. Cancer Science, 98(10), 1512–1520. doi:10.1111/j.1349-7006.2007.00550.x.
Lo, H. W., Hsu, S. C., Xia, W., Cao, X., Shih, J. Y., Wei, Y., et al. (2007). Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial–mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Research, 67(19), 9066–9076. doi:10.1158/0008-5472.CAN-07-0575.
Birchmeier, C., Birchmeier, W., Gherardi, E., & Vande Woude, G. F. (2003). Met, metastasis, motility and more. Nature Reviews Molecular Cell Biology, 4(12), 915–925. doi:10.1038/nrm1261nrm1261.
Boyer, B., & Thiery, J. P. (1993). Cyclic AMP distinguishes between two functions of acidic FGF in a rat bladder carcinoma cell line. The Journal of Cell Biology, 120(3), 767–776.
Savagner, P., Yamada, K. M., & Thiery, J. P. (1997). The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial–mesenchymal transition. The Journal of Cell Biology, 137(6), 1403–1419.
Bellusci, S., Moens, G., Thiery, J. P., & Jouanneau, J. (1994). A scatter factor-like factor is produced by a metastatic variant of a rat bladder carcinoma cell line. Journal of Cell Science, 107(Pt 5), 1277–1287.
Grotegut, S., von Schweinitz, D., Christofori, G., & Lehembre, F. (2006). Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO Journal, 25(15), 3534–3545. doi:10.1038/sj.emboj.7601213.
Min, C., Eddy, S. F., Sherr, D. H., & Sonenshein, G. E. (2008). NF-kappaB and epithelial to mesenchymal transition of cancer. Journal of Cellular Biochemistry, 104(3), 733–744. doi:10.1002/jcb.21695.
Taylor, M. A., Parvani, J. G., & Schiemann, W. P. (2010). The pathophysiology of epithelial–mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. Journal of Mammary Gland Biology and Neoplasia, 15(2), 169–190. doi:10.1007/s10911-010-9181-1.
Vincan, E., & Barker, N. (2008). The upstream components of the Wnt signalling pathway in the dynamic EMT and MET associated with colorectal cancer progression. Clinical & Experimental Metastasis, 25(6), 657–663. doi:10.1007/s10585-008-9156-4.
Wang, Z., Li, Y., Kong, D., & Sarkar, F. H. (2010). The role of Notch signaling pathway in epithelial–mesenchymal transition (EMT) during development and tumor aggressiveness. Current Drug Targets, 11(6), 745–751.
Wendt, M. K., Allington, T. M., & Schiemann, W. P. (2009). Mechanisms of the epithelial–mesenchymal transition by TGF-beta. Future Oncology, 5(8), 1145–1168. doi:10.2217/fon.09.90.
Tian, M., & Schiemann, W. P. (2009). The TGF-beta paradox in human cancer: an update. Future Oncology, 5(2), 259–271. doi:10.2217/14796694.5.2.259.
Tian, F., DaCosta Byfield, S., Parks, W. T., Yoo, S., Felici, A., Tang, B., et al. (2003). Reduction in Smad2/3 signaling enhances tumorigenesis but suppresses metastasis of breast cancer cell lines. Cancer Research, 63(23), 8284–8292.
Tian, F., Byfield, S. D., Parks, W. T., Stuelten, C. H., Nemani, D., Zhang, Y. E., et al. (2004). Smad-binding defective mutant of transforming growth factor beta type I receptor enhances tumorigenesis but suppresses metastasis of breast cancer cell lines. Cancer Research, 64(13), 4523–4530. doi:10.1158/0008-5472.CAN-04-003064/13/4523.
Deckers, M., van Dinther, M., Buijs, J., Que, I., Lowik, C., van der Pluijm, G., et al. (2006). The tumor suppressor Smad4 is required for transforming growth factor beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Research, 66(4), 2202–2209. doi:10.1158/0008-5472.CAN-05-3560.
Kang, Y., He, W., Tulley, S., Gupta, G. P., Serganova, I., Chen, C. R., et al. (2005). Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proceedings of the National Academy of Sciences of the United States of America, 102(39), 13909–13914. doi:10.1073/pnas.0506517102.
Hayashi, H., Abdollah, S., Qiu, Y., Cai, J., Xu, Y. Y., Grinnell, B. W., et al. (1997). The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell, 89(7), 1165–1173.
Souchelnytskyi, S., Nakayama, T., Nakao, A., Moren, A., Heldin, C. H., Christian, J. L., et al. (1998). Physical and functional interaction of murine and Xenopus Smad7 with bone morphogenetic protein receptors and transforming growth factor-beta receptors. Journal of Biological Chemistry, 273(39), 25364–25370.
Azuma, H., Ehata, S., Miyazaki, H., Watabe, T., Maruyama, O., Imamura, T., et al. (2005). Effect of Smad7 expression on metastasis of mouse mammary carcinoma JygMC(A) cells. Journal of the National Cancer Institute, 97(23), 1734–1746. doi:10.1093/jnci/dji399.
Leivonen, S. K., Ala-Aho, R., Koli, K., Grenman, R., Peltonen, J., & Kahari, V. M. (2006). Activation of Smad signaling enhances collagenase-3 (MMP-13) expression and invasion of head and neck squamous carcinoma cells. Oncogene, 25(18), 2588–2600. doi:10.1038/sj.Onc.1209291.
Leivonen, S. K., & Kahari, V. M. (2007). Transforming growth factor-beta signaling in cancer invasion and metastasis. International Journal of Cancer, 121(10), 2119–2124. doi:10.1002/ijc.23113.
Javelaud, D., Mohammad, K. S., McKenna, C. R., Fournier, P., Luciani, F., Niewolna, M., et al. (2007). Stable overexpression of Smad7 in human melanoma cells impairs bone metastasis. Cancer Research, 67(5), 2317–2324. doi:10.1158/0008-5472.CAN-06-3950.
Janda, E., Lehmann, K., Killisch, I., Jechlinger, M., Herzig, M., Downward, J., et al. (2002). Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. The Journal of Cell Biology, 156(2), 299–313. doi:10.1083/jcb.200109037jcb.200109037.
Xie, L., Law, B. K., Chytil, A. M., Brown, K. A., Aakre, M. E., & Moses, H. L. (2004). Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia, 6(5), 603–610. doi:10.1593/neo.04241.
Neil, J. R., Johnson, K. M., Nemenoff, R. A., & Schiemann, W. P. (2008). Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-beta through a PGE2-dependent mechanisms. Carcinogenesis, 29(11), 2227–2235. doi:10.1093/carcin/bgn202.
Bhowmick, N. A., Zent, R., Ghiassi, M., McDonnell, M., & Moses, H. L. (2001). Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. Journal of Biological Chemistry, 276(50), 46707–46713. doi:10.1074/jbc.M106176200M106176200.
Galliher, A. J., & Schiemann, W. P. (2006). Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial–mesenchymal transition in mammary epithelial cells. Breast Cancer Research, 8(4), R42. doi:10.1186/bcr1524.
Galliher-Beckley, A. J., & Schiemann, W. P. (2008). Grb2 binding to Tyr284 in TbetaR-II is essential for mammary tumor growth and metastasis stimulated by TGF-beta. Carcinogenesis, 29(2), 244–251. doi:10.1093/carcin/bgm245.
Miele, L. (2006). Notch signaling. Clinical Cancer Research, 12(4), 1074–1079. doi:10.1158/1078-0432.CCR-05-2570.
Miele, L., & Osborne, B. (1999). Arbiter of differentiation and death: Notch signaling meets apoptosis. Journal of Cellular Physiology, 181(3), 393–409. doi:10.1002/(SICI)1097-4652(199912)181:3<393::AID-JCP3>3.0.CO;2-6 [pii]10.1002/(SICI)1097-4652(199912)181:3<393::AID-JCP3>3.0.CO;2-6.
Niessen, K., Fu, Y., Chang, L., Hoodless, P. A., McFadden, D., & Karsan, A. (2008). Slug is a direct Notch target required for initiation of cardiac cushion cellularization. The Journal of Cell Biology, 182(2), 315–325. doi:10.1083/jcb.200710067.
Zavadil, J., Cermak, L., Soto-Nieves, N., & Bottinger, E. P. (2004). Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO Journal, 23(5), 1155–1165. doi:10.1038/sj.emboj.76000697600069.
Niimi, H., Pardali, K., Vanlandewijck, M., Heldin, C. H., & Moustakas, A. (2007). Notch signaling is necessary for epithelial growth arrest by TGF-beta. The Journal of Cell Biology, 176(5), 695–707. doi:10.1083/jcb.200612129.
Jechlinger, M., Sommer, A., Moriggl, R., Seither, P., Kraut, N., Capodiecci, P., et al. (2006). Autocrine PDGFR signaling promotes mammary cancer metastasis. The Journal of Clinical Investigation, 116(6), 1561–1570. doi:10.1172/JCI24652.
Fischer, A. N., Fuchs, E., Mikula, M., Huber, H., Beug, H., & Mikulits, W. (2007). PDGF essentially links TGF-beta signaling to nuclear beta-catenin accumulation in hepatocellular carcinoma progression. Oncogene, 26(23), 3395–3405. doi:10.1038/sj.onc.1210121.
Gotzmann, J., Fischer, A. N., Zojer, M., Mikula, M., Proell, V., Huber, H., et al. (2006). A crucial function of PDGF in TGF-beta-mediated cancer progression of hepatocytes. Oncogene, 25(22), 3170–3185. doi:10.1038/sj.onc.1209083.
Zhao, J. H., Luo, Y., Jiang, Y. G., He, D. L., & Wu, C. T. (2011). Knockdown of beta-catenin through shRNA cause a reversal of EMT and metastatic phenotypes induced by HIF-1alpha. Cancer Investigation, 29(6), 377–382. doi:10.3109/07357907.2010.512595.
Cheng, Z. X., Sun, B., Wang, S. J., Gao, Y., Zhang, Y. M., Zhou, H. X., et al. (2011). Nuclear factor-kappaB-dependent epithelial to mesenchymal transition induced by HIF-1alpha activation in pancreatic cancer cells under hypoxic conditions. PLoS One, 6(8), e23752. doi:10.1371/journal.pone.0023752PONE-D-11-07211.
Batlle, E., Sancho, E., Franci, C., Dominguez, D., Monfar, M., Baulida, J., et al. (2000). The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nature Cell Biology, 2(2), 84–89. doi:10.1038/35000034.
Cano, A., Perez-Moreno, M. A., Rodrigo, I., Locascio, A., Blanco, M. J., del Barrio, M. G., et al. (2000). The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nature Cell Biology, 2(2), 76–83. doi:10.1038/35000025.
Hajra, K. M., Chen, D. Y., & Fearon, E. R. (2002). The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Research, 62(6), 1613–1618.
Comijn, J., Berx, G., Vermassen, P., Verschueren, K., van Grunsven, L., Bruyneel, E., et al. (2001). The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Molecular Cell, 7(6), 1267–1278. doi:S1097-2765(01)00260-X.
Eger, A., Aigner, K., Sonderegger, S., Dampier, B., Oehler, S., Schreiber, M., et al. (2005). DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene, 24(14), 2375–2385. doi:10.1038/sj.onc.1208429.
Remacle, J. E., Kraft, H., Lerchner, W., Wuytens, G., Collart, C., Verschueren, K., et al. (1999). New mode of DNA binding of multi-zinc finger transcription factors: deltaEF1 family members bind with two hands to two target sites. EMBO Journal, 18(18), 5073–5084. doi:10.1093/emboj/18.18.5073.
Yang, J., Mani, S. A., Donaher, J. L., Ramaswamy, S., Itzykson, R. A., Come, C., et al. (2004). Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell, 117(7), 927–939. doi:10.1016/j.cell.2004.06.006S0092867404005768.
Katoh, M. (2004). Human FOX gene family (review). International Journal of Oncology, 25(5), 1495–1500.
Mani, S. A., Yang, J., Brooks, M., Schwaninger, G., Zhou, A., Miura, N., et al. (2007). Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proceedings of the National Academy of Sciences of the United States of America, 104(24), 10069–10074. doi:10.1073/pnas.0703900104.
Bloushtain-Qimron, N., Yao, J., Snyder, E. L., Shipitsin, M., Campbell, L. L., Mani, S. A., et al. (2008). Cell type-specific DNA methylation patterns in the human breast. Proceedings of the National Academy of Sciences of the United States of America, 105(37), 14076–14081. doi:10.1073/pnas.0805206105.
Ray, P. S., Wang, J., Qu, Y., Sim, M. S., Shamonki, J., Bagaria, S. P., et al. (2010). FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Research, 70(10), 3870–3876. doi:10.1158/0008-5472.CAN-09-4120.
Feuerborn, A., Srivastava, P. K., Kuffer, S., Grandy, W. A., Sijmonsma, T. P., Gretz, N., et al. (2011). The Forkhead factor FoxQ1 influences epithelial differentiation. Journal of Cellular Physiology, 226(3), 710–719. doi:10.1002/jcp. 22385.
Qiao, Y., Jiang, X., Lee, S. T., Karuturi, R. K., Hooi, S. C., & Yu, Q. (2011). FOXQ1 regulates epithelial–mesenchymal transition in human cancers. Cancer Research, 71(8), 3076–3086. doi:10.1158/0008-5472.CAN-10-2787.
Zhang, H., Meng, F., Liu, G., Zhang, B., Zhu, J., Wu, F., et al. (2011). Forkhead transcription factor foxq1 promotes epithelial–mesenchymal transition and breast cancer metastasis. Cancer Research, 71(4), 1292–1301. doi:10.1158/0008-5472.CAN-10-2825.
Tang, Y., Shu, G., Yuan, X., Jing, N., & Song, J. (2011). FOXA2 functions as a suppressor of tumor metastasis by inhibition of epithelial-to-mesenchymal transition in human lung cancers. Cell Research, 21(2), 316–326. doi:10.1038/cr.2010.126.
Yori, J. L., Johnson, E., Zhou, G., Jain, M. K., & Keri, R. A. (2010). Kruppel-like factor 4 inhibits epithelial-to-mesenchymal transition through regulation of E-cadherin gene expression. Journal of Biological Chemistry, 285(22), 16854–16863. doi:10.1074/jbc.M110.114546.
Gumireddy, K., Li, A., Gimotty, P. A., Klein-Szanto, A. J., Showe, L. C., Katsaros, D., et al. (2009). KLF17 is a negative regulator of epithelial–mesenchymal transition and metastasis in breast cancer. Nature Cell Biology, 11(11), 1297–1304. doi:10.1038/ncb1974.
Wang, X., Zheng, M., Liu, G., Xia, W., McKeown-Longo, P. J., Hung, M. C., et al. (2007). Kruppel-like factor 8 induces epithelial to mesenchymal transition and epithelial cell invasion. Cancer Research, 67(15), 7184–7193. doi:10.1158/0008-5472.CAN-06-4729.
Holian, J., Qi, W., Kelly, D. J., Zhang, Y., Mreich, E., Pollock, C. A., et al. (2008). Role of Kruppel-like factor 6 in transforming growth factor-beta1-induced epithelial–mesenchymal transition of proximal tubule cells. American Journal of Physiology. Renal Physiology, 295(5), F1388–F1396. doi:10.1152/ajprenal.00055.2008.
Yu, M., Smolen, G. A., Zhang, J., Wittner, B., Schott, B. J., Brachtel, E., et al. (2009). A developmentally regulated inducer of EMT, LBX1, contributes to breast cancer progression. Genes & Development, 23(15), 1737–1742. doi:10.1101/gad.1809309.
Evdokimova, V., Tognon, C., Ng, T., Ruzanov, P., Melnyk, N., Fink, D., et al. (2009). Translational activation of snail1 and other developmentally regulated transcription factors by YB-1 promotes an epithelial–mesenchymal transition. Cancer Cell, 15(5), 402–415. doi:10.1016/j.ccr.2009.03.017.
Fernando, R. I., Litzinger, M., Trono, P., Hamilton, D. H., Schlom, J., & Palena, C. (2010). The T-box transcription factor Brachyury promotes epithelial–mesenchymal transition in human tumor cells. The Journal of Clinical Investigation, 120(2), 533–544. doi:10.1172/JCI38379.
Beltran, M., Puig, I., Pena, C., Garcia, J. M., Alvarez, A. B., Pena, R., et al. (2008). A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial–mesenchymal transition. Genes & Development, 22(6), 756–769. doi:10.1101/gad.455708.
Cano, A., & Nieto, M. A. (2008). Non-coding RNAs take centre stage in epithelial-to-mesenchymal transition. Trends in Cell Biology, 18(8), 357–359. doi:10.1016/j.tcb.2008.05.005.
Gregory, P. A., Bert, A. G., Paterson, E. L., Barry, S. C., Tsykin, A., Farshid, G., et al. (2008). The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nature Cell Biology, 10(5), 593–601. doi:10.1038/ncb1722.
Gregory, P. A., Bracken, C. P., Bert, A. G., & Goodall, G. J. (2008). MicroRNAs as regulators of epithelial–mesenchymal transition. Cell Cycle, 7(20), 3112–3118.
Park, S. M., Gaur, A. B., Lengyel, E., & Peter, M. E. (2008). The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes & Development, 22(7), 894–907. doi:10.1101/gad.1640608.
Gebeshuber, C. A., Zatloukal, K., & Martinez, J. (2009). miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Reports, 10(4), 400–405. doi:10.1038/embor.2009.9.
Kong, W., Yang, H., He, L., Zhao, J. J., Coppola, D., Dalton, W. S., et al. (2008). MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Molecular and Cellular Biology, 28(22), 6773–6784. doi:10.1128/MCB.00941-08.
Dong, P., Kaneuchi, M., Watari, H., Hamada, J., Sudo, S., Ju, J., et al. (2011). MicroRNA-194 inhibits epithelial to mesenchymal transition of endometrial cancer cells by targeting oncogene BMI-1. Molecular Cancer, 10, 99. doi:10.1186/1476-4598-10-99.
Kumarswamy, R., Mudduluru, G., Ceppi, P., Muppala, S., Kozlowski, M., Niklinski, J., et al. (2012). MicroRNA-30a inhibits epithelial-to-mesenchymal transition by targeting Snai1 and is downregulated in non-small cell lung cancer. International Journal of Cancer, 130, 2044–2053. doi:10.1002/ijc.26218.
Liu, X., Wang, C., Chen, Z., Jin, Y., Wang, Y., Kolokythas, A., et al. (2011). MicroRNA-138 suppresses epithelial–mesenchymal transition in squamous cell carcinoma cell lines. Biochemistry Journal, 440(1), 23–31. doi:10.1042/BJ20111006.
Stinson, S., Lackner, M. R., Adai, A. T., Yu, N., Kim, H. J., O’Brien, C., et al. (2011). TRPS1 targeting by miR-221/222 promotes the epithelial-to-mesenchymal transition in breast cancer. Science Signaling, 4(177), ra41. doi:10.1126/scisignal.2001538.
Aigner, A. (2011). MicroRNAs (miRNAs) in cancer invasion and metastasis: Therapeutic approaches based on metastasis-related miRNAs. J Mol Med (Berl), 89(5), 445–457. doi:10.1007/s00109-010-0716-0.
Cortez, M. A., Ivan, C., Zhou, P., Wu, X., Ivan, M., & Calin, G. A. (2010). microRNAs in cancer: From bench to bedside. Advances in Cancer Research, 108, 113–157. doi:10.1016/B978-0-12-380888-2.00004-2.
Garofalo, M., & Croce, C. M. (2011). microRNAs: Master regulators as potential therapeutics in cancer. Annual Review of Pharmacology and Toxicology, 51, 25–43. doi:10.1146/annurev-pharmtox-010510-100517.
Wright, J. A., Richer, J. K., & Goodall, G. J. (2010). microRNAs and EMT in mammary cells and breast cancer. Journal of Mammary Gland Biology and Neoplasia, 15(2), 213–223. doi:10.1007/s10911-010-9183-z.
Lombaerts, M., van Wezel, T., Philippo, K., Dierssen, J. W., Zimmerman, R. M., Oosting, J., et al. (2006). E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. British Journal of Cancer, 94(5), 661–671. doi:10.1038/sj.bjc.6602996.
Tryndyak, V. P., Beland, F. A., & Pogribny, I. P. (2010). E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. International Journal of Cancer, 126(11), 2575–2583.
Vrba, L., Jensen, T. J., Garbe, J. C., Heimark, R. L., Cress, A. E., Dickinson, S., et al. (2010). Role for DNA methylation in the regulation of miR-200c and miR-141 expression in normal and cancer cells. PLoS One, 5(1), e8697.
Ke, X. S., Qu, Y., Cheng, Y., Li, W. C., Rotter, V., Oyan, A. M., et al. (2010). Global profiling of histone and DNA methylation reveals epigenetic-based regulation of gene expression during epithelial to mesenchymal transition in prostate cells. BMC Genomics, 11, 669. doi:10.1186/1471-2164-11-669.
Bernstein, B. E., Mikkelsen, T. S., Xie, X., Kamal, M., Huebert, D. J., Cuff, J., et al. (2006). A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell, 125(2), 315–326. doi:10.1016/j.cell.2006.02.041.
Cedar, H., & Bergman, Y. (2009). Linking DNA methylation and histone modification: patterns and paradigms. Nature Reviews Genetics, 10(5), 295–304. doi:10.1038/nrg2540.
Kaimori, A., Potter, J. J., Choti, M., Ding, Z., Mezey, E., & Koteish, A. A. (2010). Histone deacetylase inhibition suppresses the transforming growth factor beta1-induced epithelial-to-mesenchymal transition in hepatocytes. Hepatology, 52(3), 1033–1045.
Yoshikawa, M., Hishikawa, K., Marumo, T., & Fujita, T. (2007). Inhibition of histone deacetylase activity suppresses epithelial-to-mesenchymal transition induced by TGF-beta1 in human renal epithelial cells. Journal of the American Society of Nephrology, 18(1), 58–65.
Jordan, N. V., Johnson, G. L., & Abell, A. N. (2011). Tracking the intermediate stages of epithelial–mesenchymal transition in epithelial stem cells and cancer. Cell Cycle, 10(17), 2865–2873.
Abell, A. N., Jordan, N. V., Huang, W., Prat, A., Midland, A. A., Johnson, N. L., et al. (2011). MAP3K4/CBP-regulated H2B acetylation controls epithelial–mesenchymal transition in trophoblast stem cells. Cell Stem Cell, 8(5), 525–537.
Wels, C., Joshi, S., Koefinger, P., Bergler, H., & Schaider, H. (2011). Transcriptional activation of ZEB1 by Slug leads to cooperative regulation of the epithelial–mesenchymal transition-like phenotype in melanoma. The Journal of Investigative Dermatology, 131(9), 1877–1885. doi:10.1038/jid.2011.142.
Casas, E., Kim, J., Bendesky, A., Ohno-Machado, L., Wolfe, C. J., & Yang, J. (2011). Snail2 is an essential mediator of Twist1-induced epithelial mesenchymal transition and metastasis. Cancer Research, 71(1), 245–254. doi:10.1158/0008-5472.CAN-10-2330.
Taube, J. H., Herschkowitz, J. I., Komurov, K., Zhou, A. Y., Gupta, S., Yang, J., et al. (2010). Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proceedings of the National Academy of Sciences of the United States of America, 107(35), 15449–15454. doi:10.1073/pnas.1004900107.
Chaffer, C. L., Brennan, J. P., Slavin, J. L., Blick, T., Thompson, E. W., & Williams, E. D. (2006). Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Research, 66(23), 11271–11278. doi:10.1158/0008-5472.CAN-06-2044.
Korpal, M., Ell, B. J., Buffa, F. M., Ibrahim, T., Blanco, M. A., Celia-Terrassa, T., et al. (2011). Direct targeting of Sec23a by miR-200 s influences cancer cell secretome and promotes metastatic colonization. Nature Medicine, 17(9), 1101–1108. doi:10.1038/nm.2401.
Chao, Y. L., Shepard, C. R., & Wells, A. (2010). Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Molecular Cancer, 9, 179.
Jeschke, U., Mylonas, I., Kuhn, C., Shabani, N., Kunert-Keil, C., Schindlbeck, C., et al. (2007). Expression of E-cadherin in human ductal breast cancer carcinoma in situ, invasive carcinomas, their lymph node metastases, their distant metastases, carcinomas with recurrence and in recurrence. Anticancer Research, 27(4A), 1969–1974.
Park, D., Karesen, R., Axcrona, U., Noren, T., & Sauer, T. (2007). Expression pattern of adhesion molecules (E-cadherin, alpha-, beta-, gamma-catenin and claudin-7), their influence on survival in primary breast carcinoma, and their corresponding axillary lymph node metastasis. APMIS, 115(1), 52–65.
Christiansen, J. J., & Rajasekaran, A. K. (2006). Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Research, 66(17), 8319–8326. doi:10.1158/0008-5472.CAN-06-0410.
Garber, K. (2008). Epithelial-to-mesenchymal transition is important to metastasis, but questions remain. Journal of the National Cancer Institute, 100(4), 232–233. doi:10.1093/jnci/djn032.
Tarin, D., Thompson, E. W., & Newgreen, D. F. (2005). The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Research, 65(14), 5996–6000. doi:10.1158/0008-5472.CAN-05-0699. discussion 6000–5991.
Thompson, E. W., Newgreen, D. F., & Tarin, D. (2005). Carcinoma invasion and metastasis: a role for epithelial–mesenchymal transition? Cancer Research, 65(14), 5991–5995. doi:10.1158/0008-5472.CAN-05-0616. discussion 5995.
Aslakson, C. J., & Miller, F. R. (1992). Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Research, 52(6), 1399–1405.
Zhang, H., Meng, F., Wu, S., Kreike, B., Sethi, S., Chen, W., et al. (2011). Engagement of I-branching beta}-1, 6-N-acetylglucosaminyltransferase 2 in breast cancer metastasis and TGF-{beta signaling. Cancer Research, 71(14), 4846–4856. doi:10.1158/0008-5472.CAN-11-0414.
Lou, Y., Preobrazhenska, O., auf dem Keller, U., Sutcliffe, M., Barclay, L., McDonald, P. C., et al. (2008). Epithelial–mesenchymal transition (EMT) is not sufficient for spontaneous murine breast cancer metastasis. Developmental Dynamics, 237(10), 2755–2768. doi:10.1002/dvdy.21658.
Futterman, M. A., Garcia, A. J., & Zamir, E. A. (2011). Evidence for partial epithelial-to-mesenchymal transition (pEMT) and recruitment of motile blastoderm edge cells during avian epiboly. Developmental Dynamics, 240(6), 1502–1511. doi:10.1002/dvdy.22607.
Chao, Y., Wu, Q., Acquafondata, M., Dhir, R., & Wells, A. (2011). Partial mesenchymal to epithelial reverting transition in breast and prostate cancer metastases. Cancer Microenviron, 5, 19–28. doi:10.1007/s12307-011-0085-4.
Bruewer, M., Hopkins, A. M., Hobert, M. E., Nusrat, A., & Madara, J. L. (2004). RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. American Journal of Physiology. Cell Physiology, 287(2), C327–C335. doi:10.1152/ajpcell.00087.200400087.2004.
Hay, E. D., & Zuk, A. (1995). Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. American Journal of Kidney Diseases, 26(4), 678–690. doi:0272-6386(95)90610-X.
Pinkas, J., & Leder, P. (2002). MEK1 signaling mediates transformation and metastasis of EpH4 mammary epithelial cells independent of an epithelial to mesenchymal transition. Cancer Research, 62(16), 4781–4790.
Xue, C., Plieth, D., Venkov, C., Xu, C., & Neilson, E. G. (2003). The gatekeeper effect of epithelial–mesenchymal transition regulates the frequency of breast cancer metastasis. Cancer Research, 63(12), 3386–3394.
Mani, S. A., Guo, W., Liao, M. J., Eaton, E. N., Ayyanan, A., Zhou, A. Y., et al. (2008). The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell, 133(4), 704–715. doi:10.1016/j.cell.2008.03.027.
Morel, A. P., Lievre, M., Thomas, C., Hinkal, G., Ansieau, S., & Puisieux, A. (2008). Generation of breast cancer stem cells through epithelial–mesenchymal transition. PLoS One, 3(8), e2888. doi:10.1371/journal.pone.0002888.
Aktas, B., Tewes, M., Fehm, T., Hauch, S., Kimmig, R., & Kasimir-Bauer, S. (2009). Stem cell and epithelial–mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Research, 11(4), R46. doi:10.1186/bcr2333.
Raimondi, C., Gradilone, A., Naso, G., Vincenzi, B., Petracca, A., Nicolazzo, C., et al. (2011). Epithelial–mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Research and Treatment, 130(2), 449–455. doi:10.1007/s10549-011-1373-x.
Kallergi, G., Papadaki, M. A., Politaki, E., Mavroudis, D., Georgoulias, V., & Agelaki, S. (2011). Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Research, 13(3), R59. doi:10.1186/bcr2896.
Armstrong, A. J., Marengo, M. S., Oltean, S., Kemeny, G., Bitting, R. L., Turnbull, J. D., et al. (2011). Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Molecular Cancer Research, 9(8), 997–1007. doi:10.1158/1541-7786.MCR-10-0490.
Li, R., Liang, J., Ni, S., Zhou, T., Qing, X., Li, H., et al. (2010). A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell, 7(1), 51–63. doi:10.1016/j.stem.2010.04.014.
Samavarchi-Tehrani, P., Golipour, A., David, L., Sung, H. K., Beyer, T. A., Datti, A., et al. (2010). Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell, 7(1), 64–77. doi:10.1016/j.stem.2010.04.015.
Blick, T., Widodo, E., Hugo, H., Waltham, M., Lenburg, M. E., Neve, R. M., et al. (2008). Epithelial mesenchymal transition traits in human breast cancer cell lines. Clinical & Experimental Metastasis, 25(6), 629–642. doi:10.1007/s10585-008-9170-6.
Neve, R. M., Chin, K., Fridlyand, J., Yeh, J., Baehner, F. L., Fevr, T., et al. (2006). A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell, 10(6), 515–527. doi:10.1016/j.ccr.2006.10.008.
Sayan, A. E., Griffiths, T. R., Pal, R., Browne, G. J., Ruddick, A., Yagci, T., et al. (2009). SIP1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer. Proceedings of the National Academy of Sciences of the United States of America, 106(35), 14884–14889. doi:10.1073/pnas.0902042106.
Fuchs, B. C., Fujii, T., Dorfman, J. D., Goodwin, J. M., Zhu, A. X., Lanuti, M., et al. (2008). Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Research, 68(7), 2391–2399. doi:10.1158/0008-5472.CAN-07-2460.
Li, L. N., Zhang, H. D., Yuan, S. J., Yang, D. X., Wang, L., & Sun, Z. X. (2008). Differential sensitivity of colorectal cancer cell lines to artesunate is associated with expression of beta-catenin and E-cadherin. European Journal of Pharmacology, 588(1), 1–8. doi:10.1016/j.ejphar.2008.03.041.
Sokol, J. P., Neil, J. R., Schiemann, B. J., & Schiemann, W. P. (2005). The use of cystatin C to inhibit epithelial–mesenchymal transition and morphological transformation stimulated by transforming growth factor-beta. Breast Cancer Research, 7(5), R844–R853. doi:10.1186/bcr1312.
Feldmann, G., Dhara, S., Fendrich, V., Bedja, D., Beaty, R., Mullendore, M., et al. (2007). Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: A new paradigm for combination therapy in solid cancers. Cancer Research, 67(5), 2187–2196. doi:10.1158/0008-5472.CAN-06-3281.
Feldmann, G., Fendrich, V., McGovern, K., Bedja, D., Bisht, S., Alvarez, H., et al. (2008). An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer. Molecular Cancer Therapeutics, 7(9), 2725–2735. doi:10.1158/1535-7163.MCT-08-0573.
Yauch, R. L., Januario, T., Eberhard, D. A., Cavet, G., Zhu, W., Fu, L., et al. (2005). Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clinical Cancer Research, 11(24 Pt 1), 8686–8698. doi:10.1158/1078-0432.CCR-05-1492.
Kajiyama, H., Shibata, K., Terauchi, M., Yamashita, M., Ino, K., Nawa, A., et al. (2007). Chemoresistance to paclitaxel induces epithelial–mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. International Journal of Oncology, 31(2), 277–283.
Konecny, G. E., Venkatesan, N., Yang, G., Dering, J., Ginther, C., Finn, R., et al. (2008). Activity of lapatinib a novel HER2 and EGFR dual kinase inhibitor in human endometrial cancer cells. British Journal of Cancer, 98(6), 1076–1084. doi:10.1038/sj.bjc.6604278.
Yang, Y., Pan, X., Lei, W., Wang, J., & Song, J. (2006). Transforming growth factor-beta1 induces epithelial-to-mesenchymal transition and apoptosis via a cell cycle-dependent mechanism. Oncogene, 25(55), 7235–7244.
Vega, S., Morales, A. V., Ocana, O. H., Valdes, F., Fabregat, I., & Nieto, M. A. (2004). Snail blocks the cell cycle and confers resistance to cell death. Genes & Development, 18(10), 1131–1143.
Mejlvang, J., Kriajevska, M., Vandewalle, C., Chernova, T., Sayan, A. E., Berx, G., et al. (2007). Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Molecular Biology of the Cell, 18(11), 4615–4624.
Acknowledgment
The authors would like to thank Ms. Elizabeth A. Katz of the Barbara Ann Karmanos Cancer Institute’s Marketing & Communications Department for editing our manuscript. We also want to thank Dr. Cecilia Speyer of the Department of Surgery, WSU for her insightful discussions. This work was supported in part by the Susan G. Komen grant KG080465 (G. Wu) and the NOMIC grant from the Karmanos Cancer Institute (G. Wu).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meng, F., Wu, G. The rejuvenated scenario of epithelial–mesenchymal transition (EMT) and cancer metastasis. Cancer Metastasis Rev 31, 455–467 (2012). https://doi.org/10.1007/s10555-012-9379-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-012-9379-3