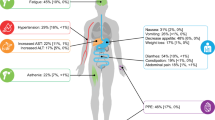
Abstract
Treatment options for metastatic renal cell carcinoma (mRCC) have evolved very rapidly, as reflected by the approval of the many drugs that have shown efficacy in phase III studies. Approved drugs include tyrosine kinase inhibitors (TKI) such as sunitinib, sorafenib and pazopanib, vascular endothelial growth factor inhibitors such as bevacizumab, and mammalian target of rapamycin (mTOR) inhibitors such as temsirolimus and everolimus. These biological agents have toxicity profiles that differ from those accompanying current chemotherapeutic agents, but their novelty leads to a lack of exhaustive clinical data regarding related adverse events (AEs), whose symptoms may overlap with those of the chronic illnesses of patients with mRCC such as hypertension, hyperglycemia, and pneumonitis. Hypertension, hypothyroidism, hand–foot syndrome, and fatigue are AEs frequently associated with TKIs; whereas immunosuppression, stomatitis, metabolic alterations, and non-infectious pneumonitis are AEs of mTOR inhibitors. Recommendations for treating these adverse events in patients with mRCC are usually the same as those for the general population. Mild to moderate toxicities may be managed with supportive and pharmacologic interventions, but higher-grade toxicities usually require external specialist consultation, dose reductions, and treatment interruption or discontinuation. Some groups of patients with mRCC, such as frail, elderly patients, and patients with renal or liver dysfunction, require special management of AEs.



Similar content being viewed by others
References
Schutzm, F. A., Je, Y., Richards, C. J., & Choueiri, T. K. (2012). Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. Journal of Clinical Oncology. Feb 6.
EMA. Sunitinib (Sutent®)—Summary of product characteristics: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000687/WC500057737.pdf (accessed on October 2011).
Motzer, R. J., Hutson, T. E., Tomczak, P., Michaelson, M. D., Bukowski, R. M., Rixe, O., et al. (2007). Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. The New England Journal of Medicine, 356(2), 115–124. Jan 11.
Rini, B. I., Tamaskar, I., Shaheen, P., Salas, R., Garcia, J., Wood, L., et al. (2007). Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. Journal of the National Cancer Institute, 99(1), 81–83. Jan 3.
Chen, A., & Agarwal, N. (2009). Reversible posterior leucoencephalopathy syndrome associated with sunitinib. Internal Medicine Journal, 39(5), 341–342.
Padhy, B. M., Shanmugam, S. P., Gupta, Y. K., & Goyal, A. (2011). Reversible posterior leucoencephalopathy syndrome in an elderly male on sunitinib therapy. British Journal of Clinical Pharmacology, 71(5), 777–779.
Marschner, N. (2010). Sunitinib in clinical practice: the expanded access program for metastatic renal cell carcinoma. Onkologie, 33(Suppl 1), 12–14.
EMA. Sorafenib (Nexavar®)—Summary of product characteristics: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000690/WC500027704.pdf (accessed on October, 2011).
Escudier, B., Eisen, T., Stadler, W. M., Szczylik, C., Oudard, S., Siebels, M., et al. (2007). Sorafenib in advanced clear-cell renal-cell carcinoma. The New England Journal of Medicine, 356(2), 125–134. Jan 11.
Beck, J., Procopio, G., Bajetta, E., Keilholz, U., Negrier, S., Szczylik, C., et al. (2011). Final results of the European Advanced Renal Cell Carcinoma Sorafenib (EU-ARCCS) expanded-access study: a large open-label study in diverse community settings. Annals of Oncology, 22(8), 1812–1823.
EMA. Pazopanib (Votrient®)—Summary of product characteristics: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001141/WC500094272.pdf (accessed on October, 2011).
Sternberg, C. N., Davis, I. D., Mardiak, J., Szczylik, C., Lee, E., Wagstaff, J., et al. (2010). Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. Journal of Clinical Oncology, 28(6), 1061–1068. Feb 20.
Rini, B. I., Escudier, B., Tomczak, P., Kaprin, A., Szczylik, C., Hutson, T. E., et al. (2011). Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. Nov 3.
EMA. Bevacizumab (Avastin®)—Summary of product characteristics: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000582/WC500029271.pdf (accessed on October 2011).
Escudier, B., Pluzanska, A., Koralewski, P., Ravaud, A., Bracarda, S., Szczylik, C., et al. (2007). Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet, 370(9605), 2103–2111. Dec 22.
EMA (Ed.) Temsirolimus (Torisel®)—Summary of product characteristics: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000799/WC500039912.pdf (accessed on October 2011).
Hudes, G., Carducci, M., Tomczak, P., Dutcher, J., Figlin, R., Kapoor, A., et al. (2007). Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England Journal of Medicine, 356(22), 2271–2281. May 31.
Maroto, J. P., Hudes, G., Dutcher, J. P., Logan, T. F., White, C. S., Krygowski, M., et al. (2011). Drug-related pneumonitis in patients with advanced renal cell carcinoma treated with temsirolimus. Journal of Clinical Oncology, 29(13), 1750–1756. May 1.
EMA. Everolimus (Afinitor®)—Summary of product characteristics: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001038/WC500022814.pdf (accessed on October, 2011).
Motzer, R. J., Escudier, B., Oudard, S., Hutson, T. E., Porta, C., Bracarda, S., et al. (2010). Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer, 116(18), 4256–4265. Sep 15.
Muriel, C., Esteban, E., Corral, N., Fonseca, P. J., Luque, M., Berros, J. P., et al. (2010). Impact of the incorporation of tyrosine kinase inhibitor agents on the treatment of patients with a diagnosis of advanced renal cell carcinoma: study based on experience at the Hospital Universitario Central de Asturias. Clinical and Translational Oncology, 12(8), 562–567.
Kollmannsberger, C., Soulieres, D., Wong, R., Scalera, A., Gaspo, R., & Bjarnason, G. (2007). Sunitinib therapy for metastatic renal cell carcinoma: recommendations for management of side effects. Canadian Urology Association Journal, 1(2 Suppl), S41–S54.
Dettmer, A. (1994). Loperamide oxide in the treatment of acute diarrhea in adults. Clinical Therapeutics, 16(6), 972–980. Nov-Dec.
Rusthoven, J., Obrien, B., & Rocchi, A. (1992). Ondansetron versus metoclopramide in the prevention of chemotherapy-induced nausea and vomiting—a metaanalysis. International Journal of Oncology, 1(4), 443–450.
Yeh, S. S., & Schuster, M. W. (2006). Megestrol acetate in cachexia and anorexia. International Journal of Nanomedicine, 1(4), 411–416.
Fraenkel, M., Ketzinel-Gilad, M., Ariav, Y., Pappo, O., Karaca, M., Castel, J., et al. (2008). mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes, 57(4), 945–957.
Neumiller, J. J., & Setter, S. M. (2009). Pharmacologic management of the older patient with type 2 diabetes mellitus. The American Journal of Geriatric Pharmacotherapy, 7(6), 324–342.
Gaw, A. (2002). A new reality: achieving cholesterol-lowering goals in clinical practice. Atherosclerosis Supplements, 2(4), 5–8. discussion -11.
Hudes, G. R. (2009). Targeting mTOR in renal cell carcinoma. Cancer, 115(10 Suppl), 2313–2320. May 15.
NCCN Clinical Pratice Guidelines in Oncology. (2011). Cancer Related Fatigue. National Comprehensive Cancer Network Web Site. http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf (accessed on 10th October).
Minton, O., Richardson, A., Sharpe, M., Hotopf, M., & Stone, P. (2008). A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. Journal of the National Cancer Institute, 100(16), 1155–1166. Aug 20.
Bukowski, R. M., Stadler, W. M., McDermott, D. F., Dutcher, J. P., Knox, J. J., Miller, W. H., Jr., et al. (2010). Safety and efficacy of sorafenib in elderly patients treated in the North American advanced renal cell carcinoma sorafenib expanded access program. Oncology, 78(5–6), 340–347.
Gore, M. E., Hariharan, S., Porta, C., Bracarda, S., Hawkins, R., Bjarnason, G. A., et al. (2011). Sunitinib in metastatic renal cell carcinoma patients with brain metastases. Cancer, 117(3), 501–509. Feb 1.
Stadler, W. M., Figlin, R. A., McDermott, D. F., Dutcher, J. P., Knox, J. J., Miller, W. H., Jr., et al. (2010). Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer, 116(5), 1272–1280. Mar 1.
Staehler, M., Haseke, N., Nuhn, P., Tullmann, C., Karl, A., Siebels, M., et al. (2011). Simultaneous anti-angiogenic therapy and single-fraction radiosurgery in clinically relevant metastases from renal cell carcinoma. BJU International, 108(5), 673–678.
Khan, G., Golshayan, A., Elson, P., Wood, L., Garcia, J., Bukowski, R., et al. (2010). Sunitinib and sorafenib in metastatic renal cell carcinoma patients with renal insufficiency. Annals of Oncology, 21(8), 1618–1622.
Gupta, S., Parsa, V., Heilbrun, L. K., Smith, D. W., Dickow, B., Heath, E., et al. (2011). Safety and efficacy of molecularly targeted agents in patients with metastatic kidney cancer with renal dysfunction. Anti-Cancer Drugs, 22(8), 794–800.
Bello, C. L., Garrett, M., Sherman, L., Smeraglia, J., Ryan, B., & Toh, M. (2010). Pharmacokinetics of sunitinib malate in subjects with hepatic impairment. Cancer Chemotherapy and Pharmacology, 66(4), 699–707.
Di Gion, P., Kanefendt, F., Lindauer, A., Scheffler, M., Doroshyenko, O., Fuhr, U., et al. (2011). Clinical pharmacokinetics of tyrosine kinase inhibitors: focus on pyrimidines, pyridines and pyrroles. Clinical Pharmacokinetics, 50(9), 551–603. Sep 1.
Acknowledgments
The authors acknowledge the support of Novartis Oncology Spain, which has facilitated the necessary meetings to evaluate and discuss all the data presented in this review, and Dr. Fernando Sánchez-Barbero from HealthCo SL (Madrid, Spain) for assistance in the preparation of this manuscript.
Conflicts of interest
The authors declare that they do not have any conflict of interest that may inappropriately influence this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Méndez-Vidal, M.J., Martínez Ortega, E., Montesa Pino, Á. et al. Management of adverse events of targeted therapies in normal and special patients with metastatic renal cell carcinoma. Cancer Metastasis Rev 31 (Suppl 1), 19–27 (2012). https://doi.org/10.1007/s10555-012-9355-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-012-9355-y