Abstract
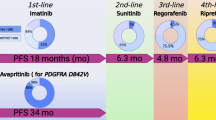
Gastrointestinal stromal tumors (GISTs) present 80% of gastrointestinal tract mesenchymal tumors, with systemic chemotherapy and radiotherapy being unable to improve survival of patients with advanced disease. The identification of activating mutations in either KIT cell surface growth factor receptor or platelet-derived growth factor receptor alpha, which lead to ligand-independent signal transduction, paved the way for the development of novel agents that selectively inhibit key molecular events in disease pathogenesis. The development of imatinib mesylate in the treatment of metastatic GIST represents a therapeutic breakthrough in molecularly targeted strategies, which crucially improved patients’ prognosis while its usefulness in adjuvant and neoadjuvant setting is under study. Sunitinib malate is available in the second-line setting, with ongoing studies evaluating its role in an earlier disease stage, while other targets are under intense investigation in order to enrich the therapeutical armamentarium for this disease. GIST phenotype seems to be an essential indicator of treatment response; thus, obtaining genotype information of each patient may be critical in order to tailor individualized treatment strategies and achieve maximal therapeutic results.


Similar content being viewed by others
References
Miettinen, M., & Lasota, J. (2001). Gastrointestinal stromal tumors—Definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archive, 438(1), 1–12.
Mazur, M. T., & Clark, H. B. (1983). Gastric stromal tumors. Reappraisal of histogenesis. American Journal of Surgical Pathology, 7(6), 507–519.
Miettinen, M., Monihan, J. M., Sarlomo-Rikala, M., Kovatich, A. J., Carr, N. J., Emory, T. S., et al. (1999). Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: Clinicopathologic and immunohistochemical study of 26 cases. American Journal of Surgical Pathology, 23(9), 1109–1118.
Syrigos, K. N., & Harrington, K. J. (2002). Targeted therapy for cancer. Oxford: Oxford University Press.
Papaetis, G. S., Roussos, C., & Syrigos, K. N. (2007). Targeted therapies for non-small cell lung cancer. Current Pharmaceutical Design, 13(27), 2810–2831.
Kindblom, L. G., Remotti, H. E., Aldenberg, F., & Meis-Kindblom, J. M. (1998). Gastrointestinal pacemaker cell tumor (GIPACT): Gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. American Journal of Pathology, 152(5), 1259–1269.
Hirota, S., Isozaki, K., Moriyama, Y., Hashimoto, K., Nishida, T., Ishiguro, S., et al. (1998). Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science, 279(5350), 577–580.
Lux, M., Rubin, B. P., Biase, T. L., Chen, C. J., Maclure, T., Demetri, G., et al. (2000). KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. American Journal of Pathology, 156(3), 791–795.
D’Amato, G., Steinert, D. M., McAuliffe, J. C., & Trent, J. C. (2005). Update on the biology and therapy of gastrointestinal stromal tumors. Cancer Control, 12(1), 44–56.
Blanke, C. D. (2006). Long-term follow-up of a phase II randomized trial in advanced gastrointestinal stromal tumor (GIST) patients (pts) treated with imatinib mesylate [abstract #9528]. Journal of Clinical Oncology, 24, 526s.
Sircar, K., Hewlett, B. R., Huizinga, J. D., Chorneyko, K., Berezin, I., & Riddell, R. H. (1999). Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. American Journal of Surgical Pathology, 23(4), 377–389.
West, R. B., Corless, C. L., Chen, X., Rubin, B. P., Subramanian, S., Montgomery, K., et al. (2004). The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. American Journal of Pathology, 165(1), 107–113.
Chirieac, L., Trent, J., & Steinert, D. M. (2003). Correlation of immunophenotype with clinical outcome of GIST patients treated with imatinib mesylate. Presented at the 9th Annual Connective Tissue Oncology Society, November 6–8, Barcelona.
Fletcher, C. D., Berman, J. J., Corless, C., Gorstein, F., Lasota, J., Longley, B. J., et al. (2002). Diagnosis of gastrointestinal stromal tumors: A consensus approach. Human Pathology, 33(5), 459–465.
Fleischman, R. A. (1993). From white spots to stem cells: The role of the Kit receptor in mammalian development. Trends in Genetics, 9(8), 285–290.
Medeiros, F., Corless, C. L., Duensing, A., Hornick, J. L., Oliveira, A. M., Heinrich, M. C., et al. (2004). KIT-negative gastrointestinal stromal tumors: Proof of concept and therapeutic implications. American Journal of Surgical Pathology, 28(7), 889–894.
Berman, J., & O’Leary, T. J. (2001). Gastrointestinal stromal tumor workshop. Human Pathology, 32(6), 578–582.
Demetri, G. D., Benjamin, R. S., Blanke, C. D., Blay, J. Y., Casali, P., Choi, H., et al. (2007). NCCN Task Force report: Management of patients with gastrointestinal stromal tumor (GIST)—Update of the NCCN clinical practice guidelines. Journal of the National Comprehensive Cancer Network, 5(Suppl 2), S1–S29.
Nilsson, B., Bumming, P., Meis-Kindblom, J. M., Oden, A., Dortok, A., Gustavsson, B., et al. (2005). Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era—A population-based study in western Sweden. Cancer, 103(4), 821–829.
DeMatteo, R., Gold, J. S., Saran, J. L., Gönen, M., Liau, K. H., Maki, R. G., et al. (2008). Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer, 112(3), 608–615.
Miettenen, M., Makhlouf, H., Sobin, L. M., & Lasota, J. (2006). Gastrointestinal stromal tumors of the jejunum and ileum: A clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. American Journal of Surgical Pathology, 30(4), 477–489.
Emory, T. S., Sobin, L. H., Lukes, L., & Emory, T. S. (1999). Prognosis of gastrointestinal smooth-muscle (stromal) tumors: Dependence on anatomic site. American Journal of Surgical Pathology, 23(1), 82–87.
Din, O. S., & Woll, P. J. (2008). Treatment of gastrointestinal stromal tumors: Focus on imatinib mesylate. Therapeutics and Clinical Risk Management, 4(1), 149–162.
Rutkowski, P., Nowecki, Z. I., Michej, W., Debiec-Rychter, M., Wozniak, A., Limon, J., et al. (2007). Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Annals of Surgical Oncology, 14(7), 2018–2027.
Takahashi, R., Tanaka, S., Kitadai, Y., Sumii, M., Yoshihara, M., Haruma, K., et al. (2003). Expression of vascular endothelial growth factor and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncology, 64(3), 266–274.
Al-Bozom, I. A. (2001). p53 expression in gastrointestinal stromal tumors. Pathology International, 51(7), 519–523.
Schneider-Stock, R., Boltze, C., Lasota, J., Miettinen, M., Peters, B., Pross, M., et al. (2003). High prognostic value of p16INK4 alterations in gastrointestinal stromal tumors. Journal of Clinical Oncology, 21(9), 1688–1697.
Debiec-Rychter, M., Lasota, J., Sarlomo-Rikala, M., Kordek, R., & Miettinen, M. (2001). Chromosomal aberrations in malignant gastrointestinal stromal tumors: Correlation with c-KIT gene mutation. Cancer Genetics and Cytogenetics, 128(1), 24–30.
Besmer, P., Murphy, J. E., George, P. C., Qiu, F., Bergold, P. J., Lederman, L., et al. (1986). A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature, 320(6061), 415–421.
Taylor, M. L., & Metcalfe, D. D. (2000). Kit signal transduction. Hematology/oncology Clinics of North America, 14(3), 517–535.
Blume-Jensen, P., Claesson-Welsh, L., Siegbahn, A., Zsebo, K. M., Westermark, B., & Heldin, C. H. (1991). Activation of the human c-kit product by ligand-induced dimerization mediates circular actin reorganization and chemotaxis. Embo Journal, 10(13), 4121–4128.
Rottapel, R., Reedijk, M., Williams, D. E., Lyman, S. D., Anderson, D. M., Pawson, T., et al. (1991). The Steel/W transduction pathway: Kit autophosphorylation and its association with a unique subset of cytoplasmic signaling proteins is induced by the Steel factor. Molecular and Cellular Biology, 11(6), 3043–3051.
Yi, T., & Ihle, J. N. (1993). Association of hematopoietic cell phosphatase with c-Kit after stimulation with c-Kit ligand. Molecular and Cellular Biology, 13(6), 3350–3358.
Blume-Jensen, P., Ronnstrand, L., Gout, I., Waterfield, M. D., & Heldin, C. H. (1994). Modulation of Kit/stem cell factor receptor-induced signaling by protein kinase C. Journal of Biological Chemistry, 269(34), 21793–21802.
Heinrich, M. C., Rubin, B. P., Longley, B. J., & Fletcher, J. A. (2002). Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Human Pathology, 33(55), 484–495.
Miettinen, M., Sobin, L. H., & Sarlomo-Rikala, M. (2000). Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Modern Pathology, 13(10), 1134–1142.
Singer, S., Rubin, B. P., Lux, M. L., Chen, C. J., Demetri, G. D., Fletcher, C. D., et al. (2002). Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. Journal of Clinical Oncology, 20(18), 3898–3905.
Lasota, J., Wozniak, A., Sarlomo-Rikala, M., Rys, J., Kordek, R., Nassar, A., et al. (2000). Mutations in exons 9 and 13 of KIT gene are rare events in gastrointestinal stromal tumors. American Journal of Pathology, 157(4), 1091–1095.
Hirota, S., Nishida, T., Isozaki, K., Taniguchi, M., Nakamura, J., Okazaki, T., et al. (2001). Gain-of-function mutation at the extracellular domain of KIT in gastrointestinal stromal tumours. Journal of Pathology, 193(4), 505–510.
Kinoshita, K., Isozaki, K., Hirota, S., Nishida, T., Chen, H., Nakahara, M., et al. (2003). c-kit gene mutation at exon 17 or 13 is very rare in sporadic gastrointestinal stromal tumors. Journal of Gastroenterology and Hepatology, 18(2), 147–151.
Antonescu, C. R., Viale, A., Sarran, L., Tschernyavsky, S. J., Gonen, M., Segal, N. H., et al. (2004). Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clinical Cancer Research, 10(10), 3282–3290.
Heinrich, M. C., Corless, C. L., Demetri, G. D., Blanke, C. D., von Mehren, M., Joensuu, H., et al. (2003). Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. Journal of Clinical Oncology, 21(23), 4342–4349.
Corless, L. C., Fletcher, J. A., & Heinrich, M. C. (2004). Biology of gastrointestinal stromal tumors. Journal of Clinical Oncology, 22(18), 3813–3825.
Kim, T. W., Lee, H., Kang, Y. K., Choe, M. S., Ryu, M. H., Chang, H. M., et al. (2004). Prognostic significance of c-kit mutation in localized gastrointestinal stromal tumors. Clinical Cancer Research, 10(9), 3076–3081.
Taniguchi, M., Nishida, T., Hirota, S., Isozaki, K., Ito, T., Nomura, T., et al. (1999). Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Research, 59(17), 4297–4300.
Isozaki, K., & Hirota, S. (2006). Gain-of-function mutations of the receptor tyrosine kinases in gastrointestinal stromal tumors. Current Genomics, 7(8), 469–475.
Martin, J., Poveda, A., Llombart-Bosch, A., Ramos, R., López-Guerrero, J. A., García del Muro, J., et al. (2005). Deletions affecting codons 557–558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: A study by the Spanish Group for Sarcoma Research (GEIS). Journal of Clinical Oncology, 23(25), 6190–6198.
Hirota, S., Ohashi, A., & Nishida, T. (2003). Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology, 125(3), 660–667.
Heinrich, M. C., Corless, C. L., Duensing, A., McGreevey, L., Chen, C. J., Joseph, N., et al. (2003). PDGFRA activating mutations in gastrointestinal stromal tumors. Science, 299(5607), 708–710.
Tornillo, L., & Terracciano, L. M. (2006). An update on molecular genetics of gastrointestinal stromal tumours. Journal of Clinical Pathology, 59(6), 557–563.
Corless, C. L., Schroeder, A., Griffith, D., Town, A., McGreevey, L., Harrell, P., et al. (2005). PDGFRA mutations in gastrointestinal stromal tumors: Frequency, spectrum and in vitro sensitivity to imatinib. Journal of Clinical Oncology, 23(23), 5357–5364.
Lasota, J., Stachura, J., & Miettinen, M. (2006). GISTs with PDGFRA exon 14 mutations represent subset of clinically favorable gastric tumors with epithelioid morphology. Laboratory Investigation, 86(1), 94–100.
Tarn, C., Rink, L., Merkel, E., Flieder, D., Pathak, H., Koumbi, D., et al. (2008). Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proceedings of the National Academy of Science, 105(24), 8387–8392.
Bauer, S., Corless, C. L., Heinrich, M. C., Dirsch, O., Antoch, G., Kanja, J., et al. (2003). Response to imatinib mesylate of a gastrointestinal stromal tumor with very low expression of KIT. Cancer Chemotherapy and Pharmacology, 51(3), 261–265.
Druker, B. J., Tamura, S., Buchdunger, E., Ohno, S., Segal, G. M., Fanning, S., et al. (1996). Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nature Medicine, 2(5), 561–566.
Tuveson, D. A., Willis, N. A., Jacks, T., Griffin, J. D., Singer, S., Fletcher, C. D., et al. (2001). STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: Biological and clinical implications. Oncogene, 20(36), 5054–5058.
Wang, W. L., Healy, M. E., Sattler, M., Verma, S., Lin, J., Maulik, G., et al. (2000). Growth inhibition and modulation of kinase pathways of small cell lung cancer cell lines by the novel tyrosine kinase inhibitor STI 571. Oncogene, 19(31), 3521–3528.
Krystal, G. W., Honsawek, S., Litz, J., & Buchdunger, E. (2000). The selective tyrosine kinase inhibitor STI571 inhibits small cell lung cancer growth. Clinical Cancer Research, 6(8), 3319–3326.
Joensuu, H., Roberts, P. J., Sarlomo-Rikala, M., Andersson, L. C., Tervahartiala, P., Tuveson, D., et al. (2001). Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. New England Journal of Medicine, 344(14), 1052–1056.
van Oosterom, A. T., Judson, I., Verweij, J., Stroobants, S., Donato di Paola, E., Dimitrijevic, S., et al. (2001). Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: A phase I study. Lancet, 358, 1421–1423.
Van Oosterom, A. T., Judson, I. R., Verweij, J., Stroobants, S., Dumez, H., Donato di Paola, E., et al. (2002). Update of phase I study of imatinib (STI571) in advanced soft tissue sarcomas and gastrointestinal stromal tumors: A report of the EORTC Soft Tissue and Bone Sarcoma Group. European Journal of Cancer, 38, S83–S87.
Van Glabbeke, M., Verweij, J., Casali, P. G., Simes, J., Le Cesne, A., Reichardt, P., et al. (2006). Predicting toxicities for patients with advanced gastrointestinal stromal tumours treated with imatinib: A study of the European Organisation for Research and Treatment of Cancer, the Italian Sarcoma Group, and the Australasian Gastro-Intestinal Trials Group (EORTC-ISG-AGITG). European Journal of Cancer, 42(16), 2277–2285.
Demetri, G. D., Von Mehren, M., Blanke, C. D., an den Abbeele, A. D., Eisenberg, B., Roberts, P. J., et al. (2002). Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. New England Journal of Medicine, 347(7), 472–480.
Dagher, R., Cohen, M., Williams, G., Rothmann, M., Gobburu, J., Robbie, G., et al. (2002). Approval summary: Imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clinical Cancer Research, 8(10), 3034–3038.
Blanke, C., Demetri, G., von Mehren, M., Heinrich, M. C., Eisenberg, B., Fletcher, J. A., et al. (2008). Long term results from a randomized phase II trial of standard-versus higher dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. Journal of Clinical Oncology, 26(4), 620–625.
Verweij, J., van Oosterom, A. T., Blay, J. Y., Judson, I., Rodenhuis, S., van der Graaf, W., et al. (2003). Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. European Journal of Cancer, 39(14), 2006–2011.
Nishida, T., Shirao, K., Sawaki, A., Koseki, M., Okamura, T., Ohtsu, A., et al. (2008). Efficacy and safety profile of imatinib mesylate (ST1571) in Japanese patients with advanced gastrointestinal stromal tumors: A phase II study (STI571B1202). International Journal of Clinical Oncology, 13(3), 244–251.
Verweij, J., Casali, P. G., Zalcberg, J., LeCesne, A., Reichardt, P., Blay, J. Y., et al. (2004). Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet, 364(9440), 1127–1134.
Casali, P. G., Verweij, J., & Kotasek, D. (2005). Imatinib mesylate in advanced gastrointestinal stromal tumors (GIST): Survival analysis of the Intergroup EORTC/ISG/AGITG randomized trial in 946 patients. European Journal of Cancer, 3, 201. suppl; abstract 711.
Sciot, R., Debiec-Rychter, M., Daugaard, S., Fisher, C., Collin, F., van Glabbeke, M., et al. (2008). Distribution and prognostic value of histopathologic data and immunohistochemical markers in gastrointestinal stromal tumours (GISTs): An analysis of the EORTC phase III trial of treatment of metastatic GISTs with imatinib mesylate. European Journal of Cancer, 44(13), 1855–1860.
Benjamin, R. S., Rankin, C., & Fletcher, C. (2003). Phase III dose-randomized study of imatinib mesylate (STI-571) for GIST: Intergroup S0033 early results. Proceedings of the Annual Meeting of the American Society of Clinical Oncology, 22, A3271.
Rankin, C., Von Mehren, M., & Blanke, C. (2004). Dose effect of imatinib (IM) in patients (pts) with metastatic GIST—Phase III Sarcoma Group Study S0033. Proceedings of the American Society of Clinical Oncology, 22, A9005.
Blanke, C. D., Rankin, C., Demetri, G. D., Ryan, C. W., von Mehren, M., Benjamin, R. S., et al. (2008). Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. Journal of Clinical Oncology, 26(4), 626–632.
Zalcberg, J. R., Verjweij, J., Casali, P. G., Le Cesne, A., Reichardt, P., Blay, J. Y., et al. (2005). Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. European Journal of Cancer, 41(12), 1751–1757.
De Giordi, U., & Verweij, J. (2005). Imatinib and gastrointestinal tumors. Where do we go from here? Molecular Cancer Therapeutics, 4(3), 495–501.
Kerkela, R., Grazette, L., Yacobi, R., Iliescu, C., Patten, R., Beahm, C., et al. (2006). Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nature Medicine, 12(8), 908–916.
Verweij, J., Casali, P. G., Kotasek, D., Le Cesne, A., Reichard, P., Judson, I. R., et al. (2007). Imatinib does not induce cardiac left ventricular failure in gastrointestinal stromal tumours patients: Analysis of EORTC-ISG-AGITG study 62005. European Journal of Cancer, 43(6), 974–978.
Debiec-Rychter, M., Sciot, R., Le Cesne, A., Schlemmer, M., Hohenberger, P., van Oosterom, A. T., et al. (2006). KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. European Journal of Cancer, 42(8), 1093–1103.
Heinrich, M. C., Owzar, K., Corless, C., Hollis, D., Borden, E. C., Fletcher, C. D., et al. (2008). Correlation of kinase genotype and clinical outcome in the North American InterGroup phase III trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 study by cancer and leukaemia group B and Southwest Oncology group. Journal of Clinical Oncology, 26(33), 5360–5367.
Van Glabbeke, M. M., Owzar, K., Rankin, C., & METAGIST, GM-AG. (2007). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors (GIST): A meta-analysis based on 1, 640 patients (pts). Journal of Clinical Oncology, ASCO Annual Meeting Proceedings, 25, 10004.
Blay, J. Y., Le Cesne, A., Ray-Coquard, I., Bui, B., Duffaud, F., Delbaldo, C., et al. (2007). Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. Journal of Clinical Oncology, 25(9), 1107–1113.
Le Cesne, A., Ray-Coquard, I., & Bui, M. (2007). Continuous versus interruption of imatinib (IM) in responding patients with advanced GIST after three years of treatment: A prospective randomized phase III trial of the French Sarcoma Group. Journal of Clinical Oncology, ASCO Annual Meeting Proceedings, 25, 10005.
Roberts, P. J., & Eisenberg, B. (2002). Clinical presentation of gastrointestinal stromal tumors and treatment of operable disease. European Journal of Cancer, 38(Suppl 5), S37–S38.
Joensuu, H., Fletcher, C., Dimitrijevic, S., Silberman, S., Roberts, P., & Demetri, G. (2002). Management of malignant gastrointestinal stromal tumors. Lancet Oncology, 3(11), 655–664.
DeMatteo, R. P. (2007). Ballman and the American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team. Adjuvant imatinib mesylate increases recurrence free survival (RFS) in patients with completely resected localized primary gastrointestinal stromal tumor (GIST): North American Intergroup Phase III trial ACOSOG Z9001. Journal of Clinical Oncology, 25(20 Suppl), 10079. Proceedings of the 43rd ASCO Annual Meeting: 1–5 June Part I.
Buemming, P., Meis-Kindblom, J. M., & Kindblom, L. G. (2003). Is there an indication for adjuvant treatment with imatinib mesylate in patients with aggressive gastrointestinal stromal tumors (GISTs)? Proceedings of the American Society of Clinical Oncology, 22, 818.
Rios, M. (2007). French Sarcoma Group: Interruption of imatinib (IM) in GIST patients with advanced disease after one year of treatment—Updated results of the prospective French Sarcoma Group randomized phase III trial on long term survival. Journal of Clinical Oncology, 25(20 Suppl), 10079. Proceedings of the 43rd ASCO Annual Meeting: 1–5 June, Part I.
Rutkowski, P., Nowecki, Z., Nyckowski, P., Dziewirski, W., Grzesiakowska, U., Nasierowska-Guttmejer, A., et al. (2006). Surgical treatment of patients with initially inoperable and/or metastatic gastrointestinal stromal tumors (GIST) during therapy with imatinib mesylate. Journal of Surgical Oncology, 93(4), 304–311.
DeMatteo, R. P., Owzar, K., Antonescu, C. R. (2008). Efficacy of adjuvant imatinib mesylate following complete resection of localized, primary gastrointestinal stromal tumor (GIST) at high risk of recurrence: The U.S. Intergroup phase II trial ACOSOG Z9000. Program and abstracts of the American Society of Clinical Oncology Gastrointestinal Cancer Symposium; January 25–27, Orlando, Florida. Abstract 8.
De Giorgi, U., Pupi, A., Turrisi, G., Montenora, I., Morini, S., Fayyaz, M., et al. (2007). Critical update and emerging trends in imatinib treatment for gastrointestinal stromal tumors. Reviews on Recent Clinical Trials, 2(1), 43–48.
Pierie, J. P., Choudry, U., Muzikansky, A., Yeap, B. Y., Souba, W. W., & Ott, M. J. (2001). The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Archives of Surgery, 136(4), 383–389.
Dematteo, R. P., Lewis, J. J., Leung, D., Mudan, S. S., Woodruff, J. M., & Brennan, M. F. (2000). Two hundred gastrointestinal stromal tumors: Recurrence patterns and prognostic factors for survival. Annals of Surgery, 231, 51–58.
Demetri, G. D. (2004). Optimal management of patients with gastrointestinal stromal tumors. Expansion and update of NCCN clinical practise guidelines. Journal of the National Comprehensive Cancer Network, 2(Suppl 1), 1–26.
Langer, C., Gunawan, B., Schuler, P., Huber, W., Fuzesi, L., & Becker, H. (2003). Prognostic factors influencing surgical management and outcome of gastrointestinal stromal tumours. British Journal of Surgery, 90(3), 332–339.
Crosby, J. A., Catton, C. N., Davis, A., Couture, J., O'Sullivan, B., Kandel, R., et al. (2001). Malignant gastrointestinal stromal tumors of the small intestine: A review of 50 cases from a prospective database. Annals of Surgical Oncology, 8(1), 50–59.
Eisenberg, B. L., & Judson, I. (2004). Surgery and imatinib in the management of GIST: Emerging approaches to adjuvant and neoadjuvant therapy. Annals of Surgical Oncology, 11(5), 465–475.
Ng, E. H., Pollock, R. E., Munsell, M. F., Atkinson, E. N., & Romsdahl, M. M. (1992). Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Annals of Surgery, 215(1), 68–77.
Yu, J., Yang, X. J., Yang, W. L., Gao, Y., & Zhang, Q. (2007). Successful resection of a giant recurrent gastrointestinal stromal tumour with imatinib mesylate as neoadjuvant therapy. Scandinavian Journal of Gastroenterology, 42(9), 1138–1140.
Loughrey, M. B., Mitchell, C., Mann, G. B., Michael, M., & Waring, P. M. (2005). Gastrointestinal stromal tumour treated with neoadjuvant imatinib. Journal of Clinical Pathology, 58(7), 779–781.
Bauer, S., Hartmann, J. T., De Wit, M., Lang, H., Grabellus, F., Antoch, G., et al. (2005). Resection of residual disease in patients with metastatic gastrointestinal stromal tumors responding to treatment with imatinib. International Journal of Cancer, 117(2), 316–325.
Raut, C. P., Posner, M., Desai, J., Morgan, J. A., George, S., Zahrieh, D., et al. (2006). Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. Journal of Clinical Oncology, 24(15), 2325–2331.
Sakakura, C., Hagiwara, A., Soga, K., Miyagawa, K., Nakashima, S., Yoshikawa, T., et al. (2006). Long-term survival of a case with multiple liver metastases from duodenal gastrointestinal stromal tumor drastically reduced by the treatment with imatinib and hepatectomy. World Journal of Gastroenterology, 12(17), 2793–2797.
Eisenberg, B. L., Harris, J., Blanke, C., Demetri, G. D., Heinrich, M. C., Watson, J. C., et al. (2008). Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): Early results of RTOG 0132/ACRIN 6665. Journal of Surgical Oncology, 99, 42–47.
Haller, F., Detken, S., Schulten, H. J., Happel, N., Gunawan, B., Kuhlgatz, J., et al. (2007). Surgical management after neoadjuvant imatinib therapy in gastrointestinal stromal tumours (GISTs) with respect to imatinib resistance caused by secondary KIT mutations. Annals of Surgical Oncology, 14(2), 526–532.
Van Glabbeke, M., Verweij, J., Casali, P. G., Le Cesne, A., Hohenberger, P., Ray-Coquard, I., et al. (2005). Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: A European Organisation of Treatment and Research of Cancer—Italian Sarcoma Group—Australasian Gastrointestinal Trials Group Study. Journal of Clinical Oncology, 23(24), 5795–5804.
Heinrich, M. C., Corless, C. L., Blanke, C. D., Demetri, G. D., Joensuu, H., Roberts, P. J., et al. (2006). Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. Journal of Clinical Oncology, 24(29), 4764–4774.
Fletcher, J. A., Corless, C. L., & Dimitrijevic, S. (2003). Mechanisms of resistance to imatinib mesylate in advanced gastrointestinal tumor. Proceedings of the American Society of Clinical Oncol, 22, 815. abstract 3275.
Wardelmann, E., Merkelbach-Bruse, S., Pauls, K., Thomas, N., Schildhaus, H. U., Heinicke, T., et al. (2006). Polyclonal evolution of multiple secondary KIT mutations in gastrointestinal stromal tumors under treatment with imatinib mesylate. Clinical Cancer Research, 12(6), 1743–1749.
Tamborini, E., Pricl, S., Negri, T., Lagonigro, M. S., Miselli, F., Greco, A., et al. (2006). Functional analyses and molecular modelling of two c-Kit mutations responsible for imatinib secondary resistance in GIST patients. Oncogene, 25(45), 6140–6146.
Chen, L. L., Trent, J. C., Wu, E. F., Fuller, G. N., Ramdas, L., Zhang, W., et al. (2004). A missense mutation in KIT kinase domain 1 correlates with imatinib resistance in gastrointestinal stromal tumors. Cancer Research, 64(17), 5913–5919.
Antonescu, C. R., Besmer, P., Guo, T., Arkun, K., Hom, G., Koryotowski, B., et al. (2005). Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clinical Cancer Research, 11(11), 4182–4190.
Debiec-Rychter, M., Cools, J., Dumez, H., Sciot, R., Stul, M., Mentens, N., et al. (2005). Mechanisms of resistance to imatinib mesylate in gastrointestinal stromal tumors and activity of PKC412 against imatinib-resistant mutants. Gastroenterology, 128(2), 270–279.
Sleijfer, S., Wiemer, E., Seynaeve, C., & Verweij, J. (2007). Improved insight into resistance mechanisms to imatinib in gastrointestinal stromal tumors: A basis for novel approaches and individualization of treatment. Oncologist, 12(6), 719–726.
Thomas, J., Wang, L., Clark, R. E., & Pirmohamed, M. (2004). Active transport of imatinib into and out of cells: Implications for drug resistance. Blood, 104(12), 3739–3745.
Burger, H., Van Tol, H., Boersma, A. W., Brok, M., Wiemer, E. A., Stoter, G., et al. (2004). Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood, 104(9), 2940–2942.
Delbaldo, C., Chatelut, E., Re, M., Deroussent, A., Séronie-Vivien, S., Jambu, A., et al. (2006). Pharmacokinetic–pharmadynamic relationships of imatinib and its main metabolite in patients with advanced gastrointestinal stromal tumors. Clinical Cancer Research, 12(20), 6073–6078.
Judson, I., Ma, P., Peng, B., Verweij, J., Racine, A., di Paola, E. D., et al. (2005). Imatinib pharmacokinetics in patients with gastrointestinal stromal tumour: A retrospective population pharmacokinetic study over time. EORT Soft Tissue and Bone Sarcoma Group. Cancer Chemotherapy and Pharmacology, 55(4), 379–386.
Gambacorti-Passerini, C., Zucchetti, M., Russo, D., Frapolli, R., Verga, M., Bungaro, S., et al. (2003). Alpha1 acid glycoprotein binds to imatinib (STI571) and substantially alters its pharmacokinetics in chronic myeloid leukemia patients. Clinical Cancer Research, 9(2), 625–632.
Mendel, D. B., Laird, A. D., Xin, X., Louie, S. G., Christensen, J. G., Li, G., et al. (2003). In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting VEGF and PDGF receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clinical Cancer Research, 9(1), 327–337.
Papaetis, G. S., Karapanagiotou, L. M., Pandha, H., & Syrigos, K. N. (2008). Targeted therapy for advanced renal cell cancer: Cytokines and beyond. Current Pharmaceutical Design, 14(22), 2229–2251.
O'Farrell, A. M., Abrams, T. J., Yuen, H. A., Ngai, T. J., Louie, S. G., Yee, K. W., et al. (2003). SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood, 101(9), 3597–3605.
Abrams, T., Lee, L. B., Murray, L. J., Pryer, N. K., & Cherrington, J. M. (2003). SU11248 inhibits KIT and platelet derived growth factor receptor-β in preclinical models of human small cell lung cancer. Molecular Cancer Therapeutics, 2(5), 471–478.
Schueneman, A. J., Himmelfarb, E., Geng, L., Tan, J., Donnelly, E., Mendel, D., et al. (2003). SU11248 maintenance therapy prevents tumor regrowth after fractionated irradiation of murine tumor models. Cancer Research, 63(14), 4009–4016.
Rosen, L., Mulay, M., & Long, J. (2003). Phase I trial of SU11248, a novel tyrosine kinase inhibitor in advanced solid tumors. Proceedings of the American Society of Clinical Oncology, 22, 765a.
Faivre, S., Delbaldo, C., Vera, K., Robert, C., Lozahic, S., Lassau, N., et al. (2006). Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. Journal of Clinical Oncology, 24(1), 25–35.
Desai, J., Yassa, L., Margusse, E., George, S., Frates, M. C., Chen, M. H., et al. (2006). Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Annals of Internal Medicine, 145(9), 660–664.
Mannavola, D., Coco, P., Vannucchi, G., Bertuelli, R., Carletto, M., Casali, P. G., et al. (2007). A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. Journal of Clinical Endocrinology and Metabolism, 92(9), 3531–3534.
Heinrichm, M. C., Maki, R., & Corless, C. L. (2006). Sunitinib (SU) response in imatinib-resistant (IM-R) GIST correlates with KIT and PDGFR mutation status. Presented at the 42nd annual meeting of the American Society for Clinical Oncology, Atlanta, GA: 2–6 June.
Morgan, J. A., Demetri, G. D., & Fletcher, J. A. (2006). Durable responses to SU11248 (sunitinib malate) are observed across all genotypes of imatinib mesylate-resistant GIST. Presented at the 17th International Congress on Anti Cancer Treatment. Paris: France, Jan 30–Feb.
Maki, R. G., Fletcher, J. A., & Heinrich, M. C. (2005). SU11248 in patients with imatinib-resistant GIST: Results from a continuation trial. American Society of Clinical Oncology. Presented at the 41st Annual Meeting; 13–17 May; Orlando, Florida, USA.
Liegl, B., Fletcher, J. A., & Corless, C. L. (2008). Correlation between KIT mutations and sunitinib (SU) resistance in GIST. Program and abstracts of the 2008 American Society of Clinical Oncology Gastrointestinal Cancer Symposium; January 25–27,Orlando, Florida. Abstract 92.
Demetri, G. D., van Oosterom, A. T., Garrett, C. R., Blackstein, M. E., Shah, M. H., Verweij, J., et al. (2006). Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumor after failure of imatinib: A randomised controlled trial. Lancet, 368(9544), 1329–1338.
Judson, I., Casali, P., & Garret, C. (2006). Updated results from a phase III trial of sunitinib in advanced gastrointestinal stromal tumors (GIST). Annals of Oncology, 17(Suppl 9), ix162.
Casali, P. G., Garret, C. R., & Blackstein, M. E. (2006). Updated results from a phase III trial of sunitinib in GIST patients (pts) for whom imatinib (IM) therapy has failed due to resistance or intolerance. Presented at the 42nd annual meeting of the American Society for Clinical Oncology, Atlanta: GA, June 2–6.
Goodman, V. L., Rock, E. P., Dagher, R., Ramchandani, R. P., Abraham, S., Jogarao, V. S., et al. (2007). Approval summary: Sunitinib for the treatment of Imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clinical Cancer Research, 13(5), 1367–1373.
George, S., Blay, J. Y., & Casali, P. G. (2008). Continuous daily dosing (CDD) of sunitinib (SU) in pts with advanced GIST: Updated efficacy, safety, PK and pharmacodynamic analysis. Journal of Clinical Oncology, 26, A10554.
Lyons, J. F., Wilhelm, S., Hibner, B., & Bollag, G. (2001). Discovery of a novel Raf kinase inhibitor. Endocrine-Related Cancer, 8(3), 219–225.
Wilhelm, S., & Chien, D. S. (2002). BAY 43-9006: Preclinical data. Current Pharmaceutical Design, 8(25), 2255–2257.
Guida, T., Anaganti, S., Provitera, L., Gedrich, R., Sullivan, E., Wilhelm, S. M., et al. (2007). Sorafenib inhibits imatinib-resistant KIT and platelet-derived growth factor receptor beta gatekeeper mutants. Clinical Cancer Research, 13(11), 3363–3369.
Guo, T., Agaram, N. P., Wong, G. C., Hom, G., D'Adamo, D., Maki, R. G., et al. (2007). Sorafenib inhibits the imatinib-resistant KITT670I gatekeeper mutation in gastrointestinal stromal tumor. Clinical Cancer Research, 13(16), 4874–4881.
Strumberg, D., Voliotis, D., Moeller, J. G., Hilger, R. A., Richly, H., Kredtke, S., et al. (2002). Results of phase I pharmacokinetic and pharmacodynamic studies of the Raf kinase inhibitor BAY 43-9006 in patients with solid tumours. International Journal of Clinical Pharmacology and Therapeutics, 40(12), 580–581.
Strumberg, D., Richly, H., Hilger, R. A., Schleucher, N., Korfee, S., Tewes, M., et al. (2005). Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. Journal of Clinical Oncology, 23(5), 965–972.
Wiebe, L., Kasza, K., & Maki, R. G. (2008). Sorafenib is active in patients with imatinib and sunitinib-resistant gastrointestinal stromal tumors (GIST): A phase II trial of the University of Chicago Phase II Consortium. Journal of Clinical Oncology, 26, 553s. Abstract 10502.
Strimpakos, A., Saif, M. W., & Syrigos, K. N. (2008). Pancreatic cancer: From molecular pathogenesis to targeted therapy. Cancer and Metastasis Reviews, 27(3), 495–522.
Drevs, J., Hofmann, I., Hugenschmidt, H., Wittig, C., Madjar, H., Muller, M., et al. (2000). Effects of PTK787/ZK 222584, a specific inhibitor of vascular endothelial growth factor receptor tyrosine kinases, on primary tumor, metastasis, vessel density, and blood flow in a murine renal cell carcinoma model. Cancer Research, 60(17), 4819–4824.
Baker, C. H., Solorzano, C. C., & Fidler, I. J. (2002). Blockade of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling for therapy of metastatic human pancreatic cancer. Cancer Research, 62(7), 1996–2003.
Thomas, A. L., Morgan, B., Horsfield, M. A., Higginson, A., Kay, A., Lee, L., et al. (2005). Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. Journal of Clinical Oncology, 23(18), 4162–4171.
Mross, K., Drevs, J., Muller, M., Medinger, M., Marme, D., Hennig, J., et al. (2005). Phase I clinical and pharmacokinetic study of PTK/ZK, a multiple VEGF receptor inhibitor, in patients with liver metastases from solid tumors. European Journal of Cancer, 41(9), 1291–1299.
Joensuu, H., De Braud, F., Coco, P., De Pas, T., Putzu, C., Spreafico, C., et al. (2008). Phase II, open-label study of PTK787/ZK222584 for the treatment of metastatic gastrointestinal stromal tumors resistant to imatinib mesylate. Annals of Oncology, 19(1), 173–177.
Weisberg, E., Manley, P. W., Breitenstein, W., Brüggen, J., Cowan-Jacob, S. W., Ray, A., et al. (2005). Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell, 7(2), 129–141.
Blay, J. Y., Casali, P. G., & Reichardt, P. (2008). A phase I study of nilotinib alone and in combination with imatinib in patients with imatinib-resistant gastrointestinal stromal tumors (GIST): Study update. Journal of Clinical Oncology, 26, 566s. Abstract 10553.
Bui, B. N., Blay, J., Duffaud, N., Hermine, O., & Le Cesne, A. (2007). Preliminary efficacy and safety results of masitinib, front line in patients with advanced GIST. A phase II study. Journal of Clinical Oncology, 26, A10025.
Lombardo, L. J., Lee, F. Y., Chen, P., Norris, D., Barrish, J. C., Behnia, K., et al. (2004). Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. Journal of Medicinal Chemistry, 47(27), 6658–6661.
Heinrich, M. C., Griffith, D. J., Druker, B. J., Wait, C. L., Ott, K. A., & Zigler, A. J. (2000). Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood, 96(3), 925–932.
Schittenhelm, M. M., Shiraga, S., Schroeder, A., Corbin, A. S., Griffith, D., Lee, F. Y., et al. (2006). Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Research, 66(1), 473–481.
Strimpakos, A. S., Karapanagiotou, E. M., Saif, W. M., & Syrigos, K. N. (2008). The role of m TOR in the management of solid tumors: An overview. Cancer Treatment Reviews, 35, 148–159.
Dumez, H., Reichard, P., & Blay, J. Y. (2008). CRAD001C2206 Study Group. A phase I-II study of everolimus (RAD001) in combination with imatinib in patients (pts) with imatinib-resistant gastrointestinal stromal tumors (GIST). Journal of Clinical Oncology, 26, 557s. Abstract 10519.
Weisberg, E., Wright, R. D., Jiang, J., Ray, A., Moreno, D., Manley, P. W., et al. (2006). Effects of PKC412, nilotinib, and imatinib against GIST-associated PDGFRA mutants with differential imatinib sensitivity. Gastroenterology, 131(6), 1734–1742.
Roberts, K. G., Odell, A. F., Byrnes, E. M., Baleato, R. M., Griffith, R., Lyons, A. B., et al. (2007). Resistance to c-KIT inhibitors conferred by V654A mutation. Molecular Cancer Therapeutics, 6(3), 1159–1166.
Xu, W., & Neckers, L. (2007). Targeting the molecular chaperone heat shock protein 90 provides a multifaceted effect on diverse cell signaling pathways of cancer cells. Clinical Cancer Research, 13(6), 1625–1629.
Mosser, D. D., & Morimoto, R. I. (2004). Molecular chaperones and the stress of oncogenesis. Oncogene, 23(16), 2907–2918.
Nanbu, K., Konishi, I., Mandai, M., Kuroda, H., Hamid, A. A., Komatsu, T., et al. (1998). Prognostic significance of heat shock proteins HSP70 and HSP90 in endometrial carcinomas. Cancer Detection and Prevention, 22(6), 549–555.
Nakatani, H., Kobayashi, M., Yin, T., Taguchi, T., Sugimoto, T., Nakano, T., et al. (2005). STI571 (Glivec) inhibits the interaction between c-KIT and heat shock protein 90 of the gastrointestinal stromal tumor cell line, GIST-T1. Cancer Science, 96(2), 116–119.
Bauer, S., Yuk, L. K., Demetri, G. D., & Fletcher, J. A. (2006). Heat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumor. Cancer Research, 66(18), 9153–9161.
Wagner, A. J., Morgan, J. A., & Chugh, R. (2008). Results from phase 1 trial of IPI-504, a novel HSP90 inhibitor, in tyrosine kinase inhibitor-resistant GIST and other sarcomas. Journal of Clinical Oncology, 26, 553s. Abstract 10503.
Dewaele, B., Wasag, B., Cools, J., Sciot, R., Prenen, H., Vandenberghe, P., et al. (2008). Activity of dasatinib, a dual SRC/ABL kinase inhibitor, and IPI-504, a heat shock protein 90 inhibitor, against gastrointestinal stromal tumor-associated PDGFRAD842V mutation. Clinical Cancer Research, 14(18), 5749–5758.
Sambol, E. B., Ambrosini, G., Geha, R. C., Kennealey, P. T., De-Carolis, P., O’connor, R., et al. (2006). Flavopiridol targets c-KIT transcription and induces apoptosis in gastrointestinal stromal tumor cells. Cancer Research, 66(11), 5858–5866.
Ikezoe, T., Yang, Y., Nishioka, C., Bandobashi, K., Nakatani, H., Taguchi, T., et al. (2006). Effect of SU11248 on gastrointestinal stromal tumor-T1 cells: Enhancement of growth inhibition via inhibition of 3-kinase/Akt/mammalian target of rapamycin signalling. Cancer Science, 97(9), 945–951.
Michael, A., Syrigos, K., & Pandha, H. (2008). Prostate cancer chemotherapy in the era of targeted therapy. Prostate Cancer and Prostatic Disease, 12, 13–16.
Syrigos, K. N., Zalonis, A., Kotteas, E., & Saif, M. W. (2008). Targeted therapy for oesophageal cancer: An overview. Cancer and Metastasis Reviews, 27(2), 273–288.
Papaetis, G., Giozos, I., & Syrigos, K. N. (2007). Targeted therapies for lung cancer: Differences amongst sexes. In V. N. Torres (Ed.), Lung cancer in women (pp. 171–193). Hauppauge: Nova Science.
Charpidou, A., Blatza, D., Anagnostou, E., & Syrigos, K. N. (2008). Review: EGFR mutations in non-small cell lung cancer—Clinical implications. In Vivo, 22(4), 529–536.
Van den Abbeele, A. D. (2008). The lessons of GIST—PET and PET/CT: A new paradigm for imaging. Oncologist, 13(suppl 2), 8–13.
Van den Abbeele, A. D. (2001). For the GIST Collaborative PET Study Group Dana-Farber Cancer Institute, Boston, Massachusetts; OHSU, Portland, Oregon, Helsinki University Central Hospital, Turku University Central Hospital, Finland, Novartis Oncology. F18-FDG-PET provides early evidence of biological response to STI571 in patients with malignant gastrointestinal stromal tumors (GIST). Proceedings of the American Society of Clinical Oncology, 20, 362a.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Papaetis, G.S., Syrigos, K.N. Targeted therapy for gastrointestinal stromal tumors: current status and future perspectives. Cancer Metastasis Rev 29, 151–170 (2010). https://doi.org/10.1007/s10555-010-9206-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-010-9206-7