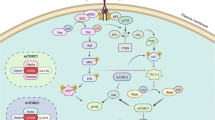
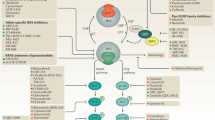
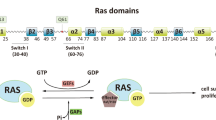
Abstract
Mutated ras has been identified in approximately 30% of human tumors, and dysregulation of ras function and signal transduction pathways is a critical step in tumorigenesis. Herein, we review the early data that supports the concept that the intrinsic radiosensitivity of tumor cells can be altered by oncogenic ras expression and that this impacts the PI3K-dependent signaling cascade. This ras-induced radioresistance can be reversed using prenyl transferase inhibitors (PTIs.). We discuss the effects of PTIs as a radiosensitizer in both in vivo and in vitro studies and show that PTIs can lead to increased radiosensitization in vivo through a variety of potential mechanisms that enhance radiation-induced cell kill. We critically evaluate the use of ras biomarkers in predicting the clinical response to PTIs that may explain the mixed results seen thus far in clinical trials using PTIs as a clinical radiosensitizer. We conclude that Ras-mediated radioresistance is the result of multiple intercommunicating pathways functioning against a complex genetic background and a solitary biomarker may not be adequate to predict for PTI-mediated radiosensitization. Nonetheless, our knowledge of the ras-signaling pathway has led to development and testing of specific therapies directed against PI3K-AKT signaling pathways as a future approach towards clinical radiosensitization.


Similar content being viewed by others
References
Barbacid, M. (1987). Ras genes. Annual Review of Biochemistry, 56, 779–827.
Barbacid, M. (1990). Ras oncogenes: Their role in neoplasia. European Journal of Clinical Investigation, 20, 225–235.
Burchill, S. A., Neal, D. E., & Lunec, J. (1994). Frequency of H-ras mutations in human bladder cancer detected by direct sequencing. British Journal of Urology, 73, 516–521.
Land, H., Parada, L., & Weinberg, R. (1983). Cellular oncogenes and multistep carcinogenesis. Science, 222, 771–778.
Garbisa, S., Pozzatti, R., Muschel, R. J., et al. (1987). Secretion of type IV collagenolytic protease and metastatic phenotype: Induction by transfection with c-Ha-ras but not c-Ha-ras plus Ad2-E1a. Cancer Research, 47, 1523–1528.
Muschel, R. J., & McKenna, W. G. (1989). Oncogenes and tumor progression. Anticancer Research, 9, 1395–1406.
Shirasawa, S., Furuse, M., Yokoyama, N., & Sasazuki, T. (1993). Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science, 260, 85–88.
Gupta, S., Plattner, R., Der, C. J., & Stanbridge, E. J. (2002). Dissection of Ras-dependent signaling pathways controlling aggressive tumor growth of human fibrosarcoma cells: Evidence for a potential novel pathway. Molecular and Cellular Biology, 20, 9294–9306.
Gupta, S., & Stanbridge, E. J. (2001). Paired human fibrosarcoma cell lines that possess or lack endogenous mutant N-ras alleles as experimental model for Ras signaling pathways. Methods in Enzymology, 333, 290–306.
Reuther, G. W., & Der, C. J. (2000). The Ras branch of small GTPases: Ras family members don't fall far from the tree. Current Opinion in Cell Biology, 12, 157–165.
Chakravarti, A., Chakladar, A., Delaney, M. A., Latham, D. E., & Loeffler, J. S. (2002). The epidermal growth factor receptor pathway mediates resistance to sequential administration of radiation and chemotherapy in primary human glioblastoma cells in a RAS-dependent manner. Cancer Research, 62, 4307–4315.
Reardon, D. B., Contessa, J. N., Mikkelsen, R. B., et al. (1999). Dominant negative EGFR-CD533 and inhibition of MAPK modify JNK1 activation and enhance radiation toxicity of human mammary carcinoma cells. Oncogene, 18, 4756–4766.
Hagan, M., Wang, L., Hanley, J. R., Park, J. S., & Dent, P. (2000). Ionizing radiation-induced mitogen-activated protein (MAP) kinase activation in DU145 prostate carcinoma cells: MAP kinase inhibition enhances radiation-induced cell killing and G2/M-phase arrest. Radiation Research, 153, 371–383.
Gupta, A. K., Bernhard, E. J., Bakanauskas, V. J., Wu, J., Muschel, R. J., & McKenna, W. G. (2000). RAS-Mediated radiation resistance is not linked to MAP kinase activation in two bladder carcinoma cell lines. Radiation Research, 154, 64–72.
Grana, T. M., Rusyn, E. V., Zhou, H., Sartor, C. I., & Cox, A. D. (2002). Ras mediates radioresistance through both phosphatidylinositol 3-kinase-dependent and Raf-dependent but mitogen-activated protein kinase/extracellular signal-regulated kinase kinase-independent signaling pathways. Cancer Research, 62, 4142–4150.
Kasid, U., Pfeifer, A., Brennan, T., et al. (1989). Effect of antisense c-raf-1 on tumorigenicity and radiation sensitivity of a human squamous carcinoma. Science, 243, 1354–1356.
Kasid, U., Pirollo, K., Dritschilo, A., & Chang, E. (1993). Oncogenic basis of radiation resistance. Advances in Cancer Research, 61, 195–233.
Gupta, A. K., Bakanauskas, V. J., Cerniglia, G. J., et al. (2001). The Ras radiation resistance pathway. Cancer Research, 61, 4278–4282.
Rodriguez-Viciana, P., Warne, P. H., Dhand, R., et al. (1994). Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature, 370, 527–532.
Kodaki, T., Woscholski, R., Hallberg, B., Rodriguez-Viciana, P., Downward, J., & Parker, P. J. (1994). The activation of phosphatidylinositol 3-kinase by Ras. Current Biology, 4, 798–806.
Rodriguez-Viciana, P., Warne, P. H., Khwaja, A., et al. (1997). Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell, 89, 457–467.
Khwaja, A., Rodriguez-Viciana, P., Wennstrom, S., Warne, P. H., & Downward, J. (1997). Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. Embo Journal, 16, 2783–2793.
Liang, K., Jin, W., Knuefermann, C., et al. (2003). Targeting the phosphatidylinositol 3-kinase/Akt pathway for enhancing breast cancer cells to radiotherapy. Molecular Cancer Therapeutics, 2, 353–360.
Gupta, A. K., Cerniglia, G. J., Mick, R., et al. (2003). Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. International Journal of Radiation Oncology, Biology, Physics, 56, 846–853.
Luo, J., Manning, B. D., & Cantley, L. C. (2003). Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cells, 4, 257–262.
Kim, I., Fernandes, A., Wu, J., et al. (2003). Selective inhibition of RAS signaling pathway increases the radiosensitivity in the wild type head and neck squamous cancer cell line with EGFR overexpression. International Journal of Radiation Oncology, Biology, Physics, 57, S354.
Kim, I. A., Fernandes, A. T., Gupta, A. K., McKenna, W. G., & Bernhard, E. J. (2004). The influence of Ras pathway signaling on tumor radiosensitivity. Cancer Metastasis Review, 23, 227–236.
Gupta, A. K., McKenna, W. G., Weber, C. N., et al. (2002). Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clinical Cancer Research, 8, 885–892.
Hancock, J. F. (2003). Ras proteins: Different signals from different locations. Nature Reviews. Molecular Cell Biology, 4, 373–384.
Hancock, J. F., Magee, A. I., Childs, J. E., & Marshall, C. J. (1989). All ras proteins are polyisoprenylated but only some are palmitoylated. Cell, 57, 1167–1177.
Hancock, J. F., Paterson, H., & Marshall, C. J. (1990). A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell, 63, 133–139.
Voice, J. K., Klemke, R. L., Le, A., & Jackson, J. H. (1999). Four human ras homologs differ in their abilities to activate Raf-1, induce transformation, and stimulate cell motility. Journal of Biological Chemistry, 274, 17164–17170.
Sieburth, D. S., Sun, Q., & Han, M. (1998). SUR-8, a conserved Ras-binding protein with leucine-rich repeats, positively regulates Ras-mediated signaling in C. elegans. Cell, 94, 119–130.
Wolfman, A. (2001). Ras isoform-specific signaling: location, location, location. Sci STKE, PE2,
Roy, S., Luetterforst, R., Harding, A., et al. (1999). Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. 1, 98–105.
Prior, I. A., Harding, A., Yan, J., Sluimer, J., Parton, R. G., & Hancock, J. F. (2001). GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nature Cell Biology, 3, 368–375.
Chen, X., & Resh, M. D. (2001). Activation of mitogen-activated protein kinase by membrane-targeted Raf chimeras is independent of raft localization. Journal of Biology Chemistry, 276, 34617–34623.
Prior, I. A., Muncke, C., Parton, R. G., & Hancock, J. F. (2003). Direct visualization of Ras proteins in spatially distinct cell surface microdomains. Journal of Cell Biology, 160, 165–170.
Niv, H., Gutman, O., Kloog, Y., & Henis, Y. I. (2002). Activated K-Ras and H-Ras display different interactions with saturable nonraft sites at the surface of live cells. Journal of Cell Biology, 157, 865–872.
FitzGerald, T. J., Daugherty, C., Kase, K., Rothstein, L. A., McKenna, M., & Greenberger, J. S. (1985). Activated human N-ras oncogene enhances x-irradiation repair of mammalian cells in vitro less effectively at low dose rate. Implications for increased therapeutic ratio of low dose rate irradiation. American Journal of Clinical Oncology, 8, 517–522.
Sklar, M. D. (1988). The ras oncogenes increase the intrinsic resistance of NIH 3T3 cells to ionizing radiation. Science, 239, 645–647.
McKenna, W. G., Weiss, M. C., Endlich, B., et al. (1990). Synergistic effect of the v-myc oncogene with H-ras on radioresistance. Cancer Research, 50, 97–102.
Bernhard, E. J., Stanbridge, E. J., Gupta, S., et al. (2000). Direct evidence for the contribution of activated N-ras and K-ras oncogenes to increased intrinsic radiation resistance in human tumor cell lines. Cancer Research, 60, 6597–6600.
Russell, J. S., Lang, F. F., Huet, T., et al. (1999). Radiosensitization of human tumor cell lines induced by the adenovirus-mediated expression of an anti-Ras single-chain antibody fragment. Cancer Research, 59, 5239–5244.
Rait, A., Pirollo, K., Will, D. W., et al. (2000). 3'-End conjugates of minimally phosphorothioate-protected oligonucleotides with 1-O-hexadecylglycerol: synthesis and anti-ras activity in radiation-resistant cells. Bioconjugate Chemistry, 11, 153–160.
Fiordalisi, J. J., Johnson 2nd, R. L., Weinbaum, C. A., et al. (2003). High affinity for farnesyltransferase and alternative prenylation contribute individually to K-Ras4B resistance to farnesyltransferase inhibitors. Journal of Biology Chemistry, 278, 41718–41727.
Capella, G., Cronauer-Mitra, S., Pienado, M. A., & Perucho, M. (1991). Frequency and spectrum of mutations at codons 12 and 13 of the c-K-ras gene in human tumors. Environmental Health Perspectives, 93, 125–131.
Kohl, N. E., Mosser, S. D., deSolms, S. J., et al. (1993). Selective inhibition of ras dependent transformation by a farnesyltransferase inhibitor. Science, 260, 1934–1936.
Prevost, G. P., Pradines, A., Viossat, I., et al. (1999). Inhibition of human tumor cell growth in vitro and in vivo by a specific inhibitor of human farnesyltransferase: BIM-46068. International Journal of Cancer, 83, 283–287.
Sepp-Lorenzino, L., Ma, Z., Rands, E., et al. (1995). A peptidomimetic inhibitor of Farnesyl:protein transferase blocks the anchorage-dependent and -independent growth of human tumor cell lines. Cancer Research, 55, 5302–5309.
Nagasu, T., Yoshimatsu, K., Rowell, C., Lewis, M. D., & Garcia, A. M. (1995). Inhibition of human tumor xenograft growth by treatment with the farnesyl transferase inhibitor B956. Cancer Research, 55, 5310–5314.
Sebti, S. M., & Der, C. J. (2003). Opinion: Searching for the elusive targets of farnesyltransferase inhibitors. Natures Review Cancer, 3, 945–951.
Yao, R., Wang, Y., Lu, Y., et al. (2006). Efficacy of the farnesyltransferase inhibitor R115777 in a rat mammary tumor model: Role of Ha-ras mutations and use of microarray analysis in identifying potential targets. Carcinogenesis, 27, 1420–1431.
Bruyneel, E. A., Storme, G. A., Schallier, D. C., Van den Berge, D. L., Hilgard, P., & Mareel, M. M. (1993). Evidence for abrogation of oncogene-induced radioresistance of mammary cancer cells by hexadecylphosphocholine in vitro. European Journal of Cancer, 29A, 1958–1963.
Miller, A. C., Gafner, J., Clark, E. P., & Samid, D. (1993). Differences in radiation-induced micronuclei yields of human cells: influence of ras gene expression and protein localization. International Journal of Radiation Biology, 64, 547–554.
Miller, A. C., Kariko, K., Myers, C. E., Clark, E. P., & Samid, D. (1993). Increased radioresistance of EJras-transformed human osteosarcoma cells and its modulation by lovastatin, an inhibitor of p21ras isoprenylation. International Journal of Cancer, 53, 302–307.
Bernhard, E. J., Kao, G., Cox, A. D., et al. (1996). The farnesyltransferase inhibitor FTI-277 radiosensitizes H-ras-transformed rat embryo fibroblasts. Cancer Research, 56, 1727–1730.
Bernhard, E. J., McKenna, W. G., Hamilton, A. D., et al. (1998). Inhibiting Ras prenylation increases the radiosensitivity of human tumor cell lines with activating mutations of ras oncogenes. Cancer Research, 58, 1754–1761.
Shi, Y., Wu, J., Mick, R., et al. (2005). Farnesyltransferase inhibitor effects on prostate tumor micro-environment and radiation survival. Prostate, 62, 69–82.
Cohen-Jonathan, E., Muschel, R. J., Gillies McKenna, W., et al. (2000). Farnesyltransferase inhibitors potentiate the antitumor effect of radiation on a human tumor xenograft expressing activated HRAS. Radiation Research, 154, 125–132.
Brunner, T. B., Cengel, K. A., Hahn, S. M., et al. (2205). Pancreatic cancer cell radiation survival and prenyltransferase inhibition: The role of K-Ras. Cancer Research, 65, 8433–8441.
Kim, J., Seong, J., & Kim, S. H. (2004). Enhancement of tumor response by farnesyltransferase inhibitor in C3H/HeJ hepatocarcinoma. Annals of the New York Academyof Sciences, 1030, 95–102.
Delmas, C., End, D., Rochaix, P., Favre, G., Toulas, C., & Cohen-Jonathan, E. (2003). The farnesyltransferase inhibitor R115777 reduces hypoxia and matrix metalloproteinase 2 expression in human glioma xenograft. Clinical Cancer Research, 9, 6062–6068.
Larner, J., Jane, J., Laws, E., Packer, R., Myers, C., & Shaffrey, M. (1998). A phase I-II trial of lovastatin for anaplastic astrocytoma and glioblastoma multiforme. American Journal of Clinical Oncology, 21, 579–583.
Hahn, S. M., Bernhard, E. J., Regine, W., et al. (2002). A phase I trial of the farnesyltransferase inhibitor L-778,123 and radiotherapy for locally advanced lung and head and neck cancer. Clinical Cancer Research, 8, 1065–1072.
Martin, N. E., Brunner, T. B., Kiel, K. D., et al. (2004). A phase I trial of the dual farnesyltransferase and geranylgeranyltransferase inhibitor L-778,123 and radiotherapy for locally advanced pancreatic cancer. Clinical Cancer Research, 10, 5447–5454.
Moyal, E. C., Laprie, A., Delannes, M., et al. (2007). Phase I trial of tipifarnib (R115777) concurrent with radiotherapy in patients with glioblastoma multiforme. International Journal Radiation Oncology Biology Physics, 68, 1396–1401.
Willett, C. G., Safran, H., Abrams, R. A., Regine, W. F., & Rich, T. A. (2003). Clinical research in pancreatic cancer: the radiation therapy oncology group trials. International Journal Radiation Oncology Biology Physics, 56, 31–37.
Su, L.-N., & Little, J. B. (1992). Transformation and radiosensitivity of human diploid skin fibroblasts transfected with activated RAS oncogene and SV40 T-antigen. Internation Journal Radiation Biology, 62, 201–210.
Grant, M. L., Bruton, R. K., Byrd, P. J., et al. (1990). Sensitivity to ionising radiation of transformed human cells containing mutant ras genes. Oncogene, 5, 1159–1164.
Ling, C. C., & Endlich, B. (1989). Radioresistance induced by oncogenic transformation. Radiation Research, 120, 267–279.
Russo, P., Loprevite, M., Cesario, A., & Ardizzoni, A. (2004). Farnesylated proteins as anticancer drug targets: from laboratory to the clinic. Current Medical Chemistry Anticancer Agents, 4, 123–138.
Zhang, Y. A., Nemunaitis, J., Samuel, S. K., Chen, P., Shen, Y., & Tong, A. W. (2006). Antitumor activity of an oncolytic adenovirus-delivered oncogene small interfering RNA. Cancer Research, 66, 9736–9743.
Iannitti, D., Dipetrillo, T., Akerman, P., et al. (2005). Erlotinib and chemoradiation followed by maintenance erlotinib for locally advanced pancreatic cancer: a phase I study. American Journal of Clinical Oncology, 28, 570–575.
Cengel, K. A., Voong, K. R., Chandrasekaran, S., et al. (2007). Oncogenic K-Ras signals through epidermal growth factor receptor and wild-type H-Ras to promote radiation survival in pancreatic and colorectal carcinoma cells. Neoplasia, 9, 341–348.
Grana, T. M., Sartor, C. I., & Cox, A. D. (2003). Epidermal growth factor receptor autocrine signaling in RIE-1 cells transformed by the Ras oncogene enhances radiation resistance. Cancer Research, 63, 7807–7814.
Toulany, M., Baumann, M., & Rodemann, H. P. (2007). Stimulated PI3K-AKT signaling mediated through ligand or radiation-induced EGFR depends indirectly, but not directly, on constitutive K-Ras activity. Molecular Cancer Research, 5, 863–872.
Caron, R. W., Yacoub, A., Zhu, X., et al. (2005). H-RAS V12-induced radioresistance in HCT116 colon carcinoma cells is heregulin dependent. Molecular Cancer Therapy, 4, 243–255.
Shintani, S., Li, C., Mihara, M., et al. (2003). Enhancement of tumor radioresponse by combined treatment with gefitinib (Iressa, ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, is accompanied by inhibition of DNA damage repair and cell growth in oral cancer. International Journal Cancer, 107, 1030–1037.
Friedmann, B., Caplin, M., Hartley, J. A., & Hochhauser, D. (2004). Modulation of DNA repair in vitro after treatment with chemotherapeutic agents by the epidermal growth factor receptor inhibitor gefitinib (ZD1839). Clinical Cancer Research, 10, 6476–6486.
Lieber, M. R., Ma, Y., Pannicke, U., & Schwarz, K. (2003). Mechanism and regulation of human non-homologous DNA end-joining. Nature Reviews. Molecular Cell Biology, 4, 712–720.
Iliakis, G., Metzger, L., Muschel, R. J., & McKenna, W. G. (1990). Induction and repair of DNA double strand breaks in radiation-resistant cells obtained by transformation of primary rat embryo cells with the oncogenes H-ras and v-myc. Cancer Research, 50, 6575–6579.
Malyapa, R. S., Wright, W. D., Taylor, Y. C., & Roti Roti, J. L. (1996). DNA supercoiling changes and nuclear matrix-associated proteins: Possible role in oncogene-mediated radioresistance. International Journal of Radiation Oncology Biology Physics, 35, 963–973.
Ayene, I. S., Bernhard, E. J., McKenna, W. G., Muschel, R. J., Krisch, R. E., & Koch, C. J. (2000). DNA as an important target in radiation-induced apoptosis of MYC and MYC plus RAS transfected rat embryo fibroblasts. International Journal of Radiation Biology, 76, 343–354.
Choe, G., Horvath, S., Cloughesy, T. F., et al. (2003). Analysis of the phosphatidylinositol 3'-kinase signaling pathway in glioblastoma patients in vivo. Cancer Research, 63, 2742–2746.
Cengel, K. A., Deutsch, E., Stephens, T. C., Voong, K. R., Kao, G. D., & Bernhard, E. J. (2006). Radiosensitizing effects of the prenyltransferase inhibitor AZD3409 against RAS mutated cell lines. Cancer Biology Therapy, 5, 1206–1210.
Lori, F., Pollard, R. B., Whitman, L., et al. (2005). Lowering the dose of hydroxyurea minimizes toxicity and maximizes anti-HIV potency. AIDS Research Human Retroviruses, 21, 263–272.
Zujewski, J., Horak, I. D., Bol, C. J., et al. (2000). Phase I and pharmacokinetic study of farnesyl protein transferase inhibitor R115777 in advanced cancer. Journal of Clinical Oncology, 18, 927–941.
Lobell, R. B., Liu, D., Buser, C. A., et al. (2002). Preclinical and clinical pharmacodynamic assessment of L-778,123, a dual inhibitor of farnesyl:protein transferase and geranylgeranyl:protein transferase type-I. Molecular Cancer Therapeutics, 1, 747–758.
Hu, W., Wu, W., Verschraegen, C. F., et al. (2003). Proteomic identification of heat shock protein 70 as a candidate target for enhancing apoptosis induced by farnesyl transferase inhibitor. Proteomics, 3, 1904–1911.
Wang, C. C., Liao, Y. P., Mischel, P. S., Iwamoto, K. S., Cacalano, N. A., & McBride, W. H. (2006). HDJ-2 as a target for radiosensitization of glioblastoma multiforme cells by the farnesyltransferase inhibitor R115777 and the role of the p53/p21 pathway. Cancer Research, 66, 6756–6762.
Lobell, R. B., Liu, D., Buser, C. A., et al. (2002). Preclinical and clinical pharmacodynamic assessment of L-778,123, a dual inhibitor of farnesyl:protein transferase and geranylgeranyl:protein transferase type-I. Molecular Cancer Therapeutics, 1, 747–758.
Zimmerman, T. M., Harlin, H., Odenike, O. M., et al. (2004). Dose-ranging pharmacodynamic study of tipifarnib (R115777) in patients with relapsed and refractory hematologic malignancies. Journal Clinical Oncology, 22, 4816–4822.
Svensson, J. P., Stalpers, L. J., Esveldt-van Lange, R. E., et al. (2006). Analysis of gene expression using gene sets discriminates cancer patients with and without late radiation toxicity. PLoS Med, 3, e422.
Bae, S. S., Cho, H., Mu, J., & Birnbaum, M. J. (2003). Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. Journal of Biological Chemistry, 278, 49530–49536.
Gupta, A. K., Cerniglia, G. J., Mick, R., McKenna, W. G., & Muschel, R. J. (2005). HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and in vivo. Cancer Research, 65, 8256–8265.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rengan, R., Cengel, K.A. & Hahn, S.M. Clinical target promiscuity: lessons from ras molecular trials. Cancer Metastasis Rev 27, 403–414 (2008). https://doi.org/10.1007/s10555-008-9133-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-008-9133-z