Abstract
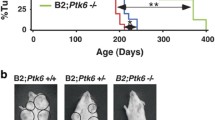
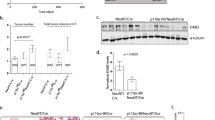
Aberrant regulation of the phosphorylation of proteins on tyrosine residues is a well-established cause of cancer. Protein tyrosine phosphatases (PTPs) share in the crucial function of maintaining appropriate levels of phosphorylation of cellular proteins, making them potentially key players in regulating the transformation process. The receptor-type tyrosine phosphatase Epsilon (RPTPɛ) participates in supporting the transformed phenotype of mammary tumor cells induced in vivo by the Neu tyrosine kinase. The phosphatase is overexpressed in mammary tumors induced in mice by a Neu transgene and expression of RPTPɛ in mouse mammary glands leads to massive hyperplasia and associated tumorigenesis. Furthermore, cells isolated from mammary tumors induced by Neu in mice genetically lacking RPTPɛ appear less transformed and proliferate less well than corresponding mammary tumor cells isolated from mice expressing the phosphatase. At the molecular level, RPTPɛ dephosphorylates and activates Src and the related kinases Yes and Fyn, and the activities of these kinases are significantly reduced in tumor cells lacking RPTPɛ. Restoring the activities of these kinases reveals that it is only the reduced activity of Src that causes the aberrant morphology and proliferation rate of tumor cells lacking RPTPɛ. RPTPɛ is primed to activate Src, and presumably related kinases, following its phosphorylation by Neu at Y695 within its C-terminus. This event is crucial in enabling RPTPɛ to activate Src, but appears not to affect the activity of RPTPɛ towards unrelated substrates. We conclude that a Neu-RPTPɛ-Src pathway exists in mouse mammary tumor cells, in which Neu phosphorylates RPTPɛ thereby driving the phosphatase to specifically activate Src family kinases and to assist in maintaining the transformed phenotype.


Similar content being viewed by others
Abbreviations
- Cyt-PTPe:
-
Non receptor isoform of PTP epsilon
- EKO:
-
PTP epsilon knockout
- MMTV:
-
Mouse Mammary Tumor Virus
- PTP:
-
protein tyrosine phosphatase
- RPTPa:
-
receptor PTP alpha
- RPTPe:
-
receptor isoform of PTP epsilon
- WT:
-
Wild -Type
References
Hunter, T. (2000). Signaling—2000 and beyond. Cell, 100, 113–127.
Schlessinger, J. (2000). Cell signaling by receptor tyrosine kinases. Cell, 103, 211–225.
Manning, G., Whyte, D. B., Martinez, R., Hunter, T., & Sudarsanam, S. (2002). The protein kinase complement of the human genome. Science, 298, 1912–1934.
Alonso, A., Sasin, J., Bottini, N., Friedberg, I., Osterman, A., Godzik, A., et al. (2004). Protein tyrosine phosphatases in the human genome. Cell, 117, 699–711.
Thomas, S. M., & Brugge, J. S. (1997). Cellular functions regulated by Src family kinases. Annual Review of Cell and Developmental Biology, 13, 513–609.
Wu, J., & Luo, H. (2005). Recent advances on T-cell regulation by receptor tyrosine kinases. Current Opinion in Hematology, 12, 292–297.
Denu, J. M., & Dixon, J. E. (1998). Protein tyrosine phosphatases: mechanisms of catalysis and regulation. Current Opinion in Chemical Biology, 2, 633–641.
Barford, D., Das, A. K., & Egloff, M. P. (1998). The structure and mechanism of protein phosphatases: Insights into catalysis and regulation. Annual Review of Biophysics and Biomolecular Structure, 27, 133–164.
Wang, W. Q., Sun, J. P., & Zhang, Z. Y. (2003). An overview of the protein tyrosine phosphatase superfamily. Current Topics in Medicinal Chemistry, 3, 739–748.
Andersen, J. N., Jansen, P. G., Echwald, S. M., Mortensen, O. H., Fukada, T., Del Vecchio, R., et al. (2004). A genomic perspective on protein tyrosine phosphatases: gene structure, pseudogenes, and genetic disease linkage. FASEB Journal, 18, 8–30.
Andersen, J. N., Mortensen, O. H., Peters, G. H., Drake, P. G., Iversen, L. F., Olsen, O. H., et al. (2001). Structural and evolutionary relationships among protein tyrosine phosphatase domains. Molecular and Cellular Biology, 21, 7117–7136.
Wang, Y., & Pallen, C. J. (1991). The receptor-like protein tyrosine phosphatase HPTP alpha has two active catalytic domains with distinct substrate specificities. EMBO Journal, 10, 3231–3237.
Wu, L., Buist, A., den Hertog, J., & Zhang, Z. Y. (1997). Comparative kinetic analysis and substrate specificity of the tandem catalytic domains of the receptor-like protein-tyrosine phosphatase alpha. Journal of Biological Chemistry, 272, 6994–7002.
den Hertog, J., Blanchetot, C., Buist, A., Overvoorde, J., van der Sar, A., & Tertoolen, L. G. (1999). Receptor protein-tyrosine phosphatase signalling in development. International Journal of Developmental Biology, 43, 723–733.
Jiang, G., den Hertog, J., & Hunter, T. (2000). Receptor-like protein tyrosine phosphatase alpha homodimerizes on the cell surface. Molecular and Cellular Biology, 20, 5917–5929.
Jiang, G., den Hertog, J., Su, J., Noel, J., Sap, J., & Hunter, T. (1999). Dimerization inhibits the activity of receptor-like protein-tyrosine phosphatase-alpha. Nature, 401, 606–610.
Meng, K., Rodriguez-Pena, A., Dimitrov, T., Chen, W., Yamin, M., Noda, M., et al. (2000). Pleiotrophin signals increased tyrosine phosphorylation of beta beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta. Proceedings of the National Academy of Sciences of the United States of America, 97, 2603–2608.
Xu, Z., & Weiss, A. (2002). Negative regulation of CD45 by differential homodimerization of the alternatively spliced isoforms. Nature Immunology, 3, 764–771.
Stoker, A. (2005). Methods for identifying extracellular ligands of RPTPs. Methods, 35, 80–89.
Sallee, J. L., Wittchen, E. S., & Burridge, K. (2006). Regulation of cell adhesion by protein-tyrosine phosphatases: II. Cell-cell adhesion. Journal of Biological Chemistry, 281, 16189–16192.
Frangioni, J. V., Oda, A., Smith, M., Salzman, E. W., & Neel, B. G. (1993). Calpain-catalyzed cleavage and subcellular relocation of protein phosphotyrosine phosphatase 1B (PTP-1B) in human platelets. EMBO Journal, 12, 4843–4856.
Gil-Henn, H., Volohonsky, G., & Elson, A. (2001). Regulation of protein-tyrosine phosphatases alpha and epsilon by calpain-mediated proteolytic cleavage. Journal of Biological Chemistry, 276, 31772–31779.
Chagnon, M. J., Uetani, N., & Tremblay, M. L. (2004). Functional significance of the LAR receptor protein tyrosine phosphatase family in development and diseases. Biochemistry and Cell Biology, 82, 664–675.
van der Wijk, T., Blanchetot, C., & den Hertog, J. (2005). Regulation of receptor protein-tyrosine phosphatase dimerization. Methods, 35, 73–79.
Stoker, A. W. (2005). Protein tyrosine phosphatases and signalling. Journal of Endocrinology, 185, 19–33.
Toledano-Katchalski, H., Tiran, Z., Sines, T., Shani, G., Granot-Attas, S., den Hertog, J., et al. (2003). Dimerization in vivo and inhibition of the nonreceptor form of protein tyrosine phosphatase epsilon. Molecular and Cellular Biology, 23, 5460–5471.
Tonks, N. K. (2005). Redox redux: revisiting PTPs and the control of cell signaling. Cell, 121, 667–670.
den Hertog, J., Groen, A., & van der Wijk, T. (2005). Redox regulation of protein-tyrosine phosphatases. Archives of Biochemistry and Biophysics, 434, 11–15.
Meng, T. C., Fukada, T., & Tonks, N. K. (2002). Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Molecular Cell, 9, 387–399.
Zheng, X. M., Resnick, R. J., & Shalloway, D. (2000). A phosphotyrosine displacement mechanism for activation of Src by PTPalpha. EMBO Journal, 19, 964–978.
Zheng, X. M., Resnick, R. J., & Shalloway, D. (2002). Mitotic activation of protein-tyrosine phosphatase alpha and regulation of its Src-mediated transforming activity by its sites of protein kinase C phosphorylation. Journal of Biological Chemistry, 277, 21922–21929.
Chen, M., Chen, S. C., & Pallen, C. J. (2006). Integrin-induced tyrosine phosphorylation of protein-tyrosine phosphatase-alpha is required for cytoskeletal reorganization and cell migration. Journal of Biological Chemistry, 281, 11972–11980.
Bence, K. K., Delibegovic, M., Xue, B., Gorgun, C. Z., Hotamisligil, G. S., Neel, B. G., et al. (2006). Neuronal PTP1B regulates body weight, adiposity and leptin action. Nature Medicine, 12, 917–924.
Haj, F. G., Zabolotny, J. M., Kim, Y. B., Kahn, B. B., & Neel, B. G. (2005). Liver-specific protein-tyrosine phosphatase 1B (PTP1B) re-expression alters glucose homeostasis of PTP1B-/-mice. Journal of Biological Chemistry, 280, 15038–15046.
Klaman, L. D., Boss, O., Peroni, O. D., Kim, J. K., Martino, J. L., Zabolotny, J. M., et al. (2000). Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Molecular and Cellular Biology, 20, 5479–5489.
Zabolotny, J. M., Bence-Hanulec, K. K., Stricker-Krongrad, A., Haj, F., Wang, Y., Minokoshi, Y., et al. (2002). PTP1B regulates leptin signal transduction in vivo. Developmental Cell, 2, 489–495.
Cheng, A., Uetani, N., Simoncic, P. D., Chaubey, V. P., Lee-Loy, A., McGlade, C. J., et al. (2002). Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Developmental Cell, 2, 497–503.
Elchebly, M., Payette, P., Michaliszyn, E., Cromlish, W., Collins, S., Loy, A. L., et al. (1999). Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science, 283, 1544–1548.
Ostman, A., Hellberg, C., & Bohmer, F. D. (2006). Protein-tyrosine phosphatases and cancer. Nature Reviews. Cancer, 6, 307–320.
Bourdeau, A., Dube, N., & Tremblay, M. L. (2005). Cytoplasmic protein tyrosine phosphatases, regulation and function: The roles of PTP1B and TC-PTP. Current Opinion in Cell Biology, 17, 203–209.
Schiller, K. R., & Mauro, L. J. (2005). Tyrosine phosphatases as regulators of skeletal development and metabolism. Journal of Cellular Biochemistry, 96, 262–277.
Mustelin, T., Vang, T., & Bottini, N. (2005). Protein tyrosine phosphatases and the immune response. Nature Reviews. Immunology, 5, 43–57.
Larsen, M., Tremblay, M. L., & Yamada, K. M. (2003). Phosphatases in cell-matrix adhesion and migration. Nature Reviews. Molecular Cell Biology, 4, 700–711.
Burridge, K., Sastry, S. K., & Sallee, J. L. (2006). Regulation of cell adhesion by protein-tyrosine phosphatases. I. Cell–matrix adhesion. Journal of Biological Chemistry, 281, 15593–15596.
Angers-Loustau, A., Cote, J. F., & Tremblay, M. L. (1999). Roles of protein tyrosine phosphatases in cell migration and adhesion. Biochemistry and Cell Biology, 77, 493–505.
Berman-Golan, D., & Elson, A. (2007). Neu-mediated phosphorylation of protein tyrosine phosphatase epsilon is critical for activation of Src in mammary tumor cells. Oncogene, 26, 7028–7037.
Tautz, L., Pellecchia, M., & Mustelin, T. (2006). Targeting the PTPome in human disease. Expert Opinion on Therapeutic Targets, 10, 157–177.
Taylor, S. D., & Hill, B. (2004). Recent advances in protein tyrosine phosphatase 1B inhibitors. Expert Opinion on Investigational Drugs, 13, 199–214.
Elson, A., & Leder, P. (1995). Protein-tyrosine phosphatase epsilon. An isoform specifically expressed in mouse mammary tumors initiated by v-Ha-ras OR neu. Journal of Biological Chemistry, 270, 26116–26122.
Krueger, N. X., Streuli, M., & Saito, H. (1990). Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO Journal, 9, 3241–3252.
Elson, A., & Leder, P. (1995). Identification of a cytoplasmic, phorbol ester-inducible isoform of protein tyrosine phosphatase epsilon. Proceedings of the National Academy of Sciences of the United States of America, 92, 12235–12239.
Tanuma, N., Nakamura, K., & Kikuchi, K. (1999). Distinct promoters control transmembrane and cytosolic protein tyrosine phosphatase epsilon expression during macrophage differentiation. European Journal of Biochemistry, 259, 46–54.
Nakamura, K., Mizuno, Y., & Kikuchi, K. (1996). Molecular cloning of a novel cytoplasmic protein tyrosine phosphatase PTP epsilon. Biochemical and Biophysical Research Communications, 218, 726–732.
Gil-Henn, H., Volohonsky, G., Toledano-Katchalski, H., Gandre, S., & Elson, A. (2000). Generation of novel cytoplasmic forms of protein tyrosine phosphatase epsilon by proteolytic processing and translational control. Oncogene, 19, 4375–4384.
Wabakken, T., Hauge, H., Funderud, S., & Aasheim, H. C. (2002). Characterization, expression and functional aspects of a novel protein tyrosine phosphatase epsilon isoform. Scandinavian Journal of Immunology, 56, 276–285.
Sines, T., Granot-Attas, S., Weisman-Welcher, S., & Elson, A. (2007). Association of tyrosine phosphatase epsilon with microtubules inhibits phosphatase activity and is regulated by the epidermal growth factor receptor. Molecular and Cellular Biology, 27, 7102–7112.
Tiran, Z., Peretz, A., Shinder, V., Sap, J., Attali, B., & Elson, A. (2006). PTPs Epsilon and Alpha perform specific and overlapping functions in regulation of voltage-gated potassium channels and axon myelination. Molecular Biology of the Cell, 17(10), 4330–4342.
Peretz, A., Gil-Henn, H., Sobko, A., Shinder, V., Attali, B., & Elson, A. (2000). Hypomyelination and increased activity of voltage-gated K(+) channels in mice lacking protein tyrosine phosphatase epsilon. EMBO Journal, 19, 4036–4045.
Muja, N., Lovas, G., Romm, E., Machleder, D., Ranjan, M., Gallo, V., et al. (2004). Expression of a catalytically inactive transmembrane protein tyrosine phosphatase epsilon (tm-PTP epsilon) delays optic nerve myelination. Glia, 48, 278–297.
Chiusaroli, R., Knobler, H., Luxenburg, C., Sanjay, A., Granot-Attas, S., Tiran, Z., et al. (2004). Tyrosine phosphatase epsilon is a positive regulator of osteoclast function in vitro and in vivo. Molecular Biology of the Cell, 15, 234–244.
Kollet, O., Dar, A., Shivtiel, S., Kalinkovich, A., Lapid, K., Sztainberg, Y., et al. (2006). Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nature Medicine, 12, 657–664.
Wabakken, T., Hauge, H., Finne, E. F., Wiedlocha, A., & Aasheim, H. (2002). Expression of human protein tyrosine phosphatase epsilon in leucocytes: A potential ERK pathway-regulating phosphatase. Scandinavian Journal of Immunology, 56, 195–203.
Toledano-Katchalski, H., Kraut, J., Sines, T., Granot-Attas, S., Shohat, G., Gil-Henn, H., et al. (2003). Protein tyrosine phosphatase epsilon inhibits signaling by mitogen-activated protein kinases. Molecular Cancer Research, 1, 541–550.
Tanuma, N., Shima, H., Shimada, S., & Kikuchi, K. (2003). Reduced tumorigenicity of murine leukemia cells expressing protein-tyrosine phosphatase, PTPepsilon C. Oncogene, 22, 1758–1762.
Tanuma, N., Shima, H., Nakamura, K., & Kikuchi, K. (2001). Protein tyrosine phosphatase epsilonC selectively inhibits interleukin-6- and interleukin- 10-induced JAK-STAT signaling. Blood, 98, 3030–3034.
Tanuma, N., Nakamura, K., Shima, H., & Kikuchi, K. (2000). Protein-tyrosine phosphatase PTPepsilon C inhibits Jak-STAT signaling and differentiation induced by interleukin-6 and leukemia inhibitory factor in M1 leukemia cells. Journal of Biological Chemistry, 275, 28216–28221.
Thompson, L. J., Jiang, J., Madamanchi, N., Runge, M. S., & Patterson, C. (2001). PTP-epsilon, a tyrosine phosphatase expressed in endothelium, negatively regulates endothelial cell proliferation. American Journal of Physiology. Heart and Circulatory Physiology, 281, H396–H403.
Andersen, J. N., Elson, A., Lammers, R., Romer, J., Clausen, J. T., Moller, K. B., et al. (2001). Comparative study of protein tyrosine phosphatase-epsilon isoforms: membrane localization confers specificity in cellular signalling. Biochemical Journal, 354, 581–590.
Moller, N. P., Moller, K. B., Lammers, R., Kharitonenkov, A., Hoppe, E., Wiberg, F. C., et al. (1995). Selective down-regulation of the insulin receptor signal by protein-tyrosine phosphatases alpha and epsilon. Journal of Biological Chemistry, 270, 23126–23131.
Nakagawa, Y., Aoki, N., Aoyama, K., Shimizu, H., Shimano, H., Yamada, N., et al. (2005). Receptor-type protein tyrosine phosphatase epsilon (PTPepsilonM) is a negative regulator of insulin signaling in primary hepatocytes and liver. Zoological Science, 22, 169–175.
Sully, V., Pownall, S., Vincan, E., Bassal, S., Borowski, A. H., Hart, P. H., et al. (2001). Functional abnormalities in protein tyrosine phosphatase epsilon-deficient macrophages. Biochemical and Biophysical Research Communications, 286, 184–188.
Cardiff, R. D., Sinn, E., Muller, W., & Leder, P. (1991). Transgenic oncogene mice. Tumor phenotype predicts genotype. American Journal of Pathology, 139, 495–501.
Elson, A. (1999). Protein tyrosine phosphatase epsilon increases the risk of mammary hyperplasia and mammary tumors in transgenic mice. Oncogene, 18, 7535–7542.
Muller, W. J., Sinn, E., Pattengale, P. K., Wallace, R., & Leder, P. (1988). Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell, 54, 105–115.
Gil-Henn, H., & Elson, A. (2003). Tyrosine phosphatase-epsilon activates Src and supports the transformed phenotype of Neu-induced mammary tumor cells. Journal of Biological Chemistry, 278, 15579–15586.
Flint, A. J., Tiganis, T., Barford, D., & Tonks, N. K. (1997). Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proceedings of the National Academy of Sciences of the United States of America, 94, 1680–1685.
Blanchetot, C., Chagnon, M., Dube, N., Halle, M., & Tremblay, M. L. (2005). Substrate-trapping techniques in the identification of cellular PTP targets. Methods, 35, 44–53.
Granot-Attas, S., & Elson, A. (2004). Protein tyrosine phosphatase epsilon activates Yes and Fyn in Neu-induced mammary tumor cells. Experimental Cell Research, 294, 236–243.
Zheng, X. M., Wang, Y., & Pallen, C. J. (1992). Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature, 359, 336–339.
Su, J., Muranjan, M., & Sap, J. (1999). Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Current Biology, 9, 505–511.
Ponniah, S., Wang, D. Z., Lim, K. L., & Pallen, C. J. (1999). Targeted disruption of the tyrosine phosphatase PTPalpha leads to constitutive downregulation of the kinases Src and Fyn. Current Biology, 9, 535–538.
Zheng, X. M., & Shalloway, D. (2001). Two mechanisms activate PTPalpha during mitosis. EMBO Journal, 20, 6037–6049.
Tracy, S., van der Geer, P., & Hunter, T. (1995). The receptor-like protein-tyrosine phosphatase, RPTP alpha, is phosphorylated by protein kinase C on two serines close to the inner face of the plasma membrane. Journal of Biological Chemistry, 270, 10587–10594.
den Hertog, J., Pals, C. E., Peppelenbosch, M. P., Tertoolen, L. G., de Laat, S. W., & Kruijer, W. (1993). Receptor protein tyrosine phosphatase alpha activates pp60c-src and is involved in neuronal differentiation. EMBO Journal, 12, 3789–3798.
Bodrikov, V., Leshchyns’ka, I., Sytnyk, V., Overvoorde, J., den Hertog, J., & Schachner, M. (2005). RPTPalpha is essential for NCAM-mediated p59fyn activation and neurite elongation. Journal of Cell Biology, 168, 127–139.
von Wichert, G., Jiang, G., Kostic, A., De Vos, K., Sap, J., & Sheetz, M. P. (2003). RPTP-alpha acts as a transducer of mechanical force on alphav/beta3-integrin-cytoskeleton linkages. Journal of Cell Biology, 161, 143–153.
Zeng, L., Si, X., Yu, W. P., Le, H. T., Ng, K. P., Teng, R. M., et al. (2003). PTP alpha regulates integrin-stimulated FAK autophosphorylation and cytoskeletal rearrangement in cell spreading and migration. Journal of Cell Biology, 160, 137–146.
Harder, K. W., Moller, N. P., Peacock, J. W., & Jirik, F. R. (1998). Protein-tyrosine phosphatase alpha regulates Src family kinases and alters cell-substratum adhesion. Journal of Biological Chemistry, 273, 31890–31900.
Pallen, C. J. (2003). Protein tyrosine phosphatase alpha (PTPalpha): A Src family kinase activator and mediator of multiple biological effects. Current Topics in Medicinal Chemstry, 3, 821–835.
Maksumova, L., Le, H. T., Muratkhodjaev, F., Davidson, D., Veillette, A., & Pallen, C. J. (2005). Protein tyrosine phosphatase alpha regulates Fyn activity and Cbp/PAG phosphorylation in thymocyte lipid rafts. Journal of Immunology, 175, 7947–7956.
Pera, I. L., Iuliano, R., Florio, T., Susini, C., Trapasso, F., Santoro, M., et al. (2005). The rat tyrosine phosphatase eta increases cell adhesion by activating c-Src through dephosphorylation of its inhibitory phosphotyrosine residue. Oncogene, 24, 3187–3195.
Roskoski Jr., R. (2005). Src kinase regulation by phosphorylation and dephosphorylation. Biochemical and Biophysical Research Communications, 331, 1–14.
Lau, K. H., Wu, L. W., Sheng, M. H., Amoui, M., Suhr, S. M., & Baylink, D. J. (2006). An osteoclastic protein-tyrosine phosphatase is a potential positive regulator of the c-Src protein-tyrosine kinase activity: a mediator of osteoclast activity. Journal of Cellular Biochemistry, 97, 940–955.
Ardini, E., Agresti, R., Tagliabue, E., Greco, M., Aiello, P., Yang, L. T., et al. (2000). Expression of protein tyrosine phosphatase alpha (RPTPalpha) in human breast cancer correlates with low tumor grade, and inhibits tumor cell growth in vitro and in vivo. Oncogene, 19, 4979–4987.
Bentires-Alj, M., & Neel, B. G. (2007). Protein-tyrosine phosphatase 1B is required for HER2/Neu-induced breast cancer. Cancer Research, 67, 2420–2424.
Julien, S. G., Dube, N., Read, M., Penney, J., Paquet, M., Han, Y., et al. (2007). Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary tumorigenesis and protects from lung metastasis. Nature Genetics, 39, 338–346.
Bjorge, J. D., Pang, A., & Fujita, D. J. (2000). Identification of protein-tyrosine phosphatase 1B as the major tyrosine phosphatase activity capable of dephosphorylating and activating c-Src in several human breast cancer cell lines. Journal of Biological Chemistry, 275, 41439–41446.
Bentires-Alj, M., Gil, S. G., Chan, R., Wang, Z. C., Wang, Y., Imanaka, N., et al. (2006). A role for the scaffolding adapter GAB2 in breast cancer. Nature Medicine, 12, 114–121.
Brummer, T., Schramek, D., Hayes, V. M., Bennett, H. L., Caldon, C. E., Musgrove, E. A., et al. (2006). Increased proliferation and altered growth factor dependence of human mammary epithelial cells overexpressing the Gab2 docking protein. Journal of Biological Chemistry, 281, 626–637.
Meng, S., Chen, Z., Munoz-Antonia, T., & Wu, J. (2005). Participation of both Gab1 and Gab2 in the activation of the ERK/MAPK pathway by epidermal growth factor. Biochemical Journal, 391, 143–151.
Ke, Y., Lesperance, J., Zhang, E. E., Bard-Chapeau, E. A., Oshima, R. G., Muller, W. J., et al. (2006). Conditional deletion of Shp2 in the mammary gland leads to impaired lobulo-alveolar outgrowth and attenuated Stat5 activation. Journal of Biological Chemistry, 281, 34374–34380.
Mohi, M. G., & Neel, B. G. (2007). The role of Shp2 (PTPN11) in cancer. Current Opinion in Genetics & Development, 17, 23–30.
Chan, R. J., & Feng, G. S. (2007). PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood, 109, 862–867.
Kim, H., Chan, R., Dankort, D. L., Zuo, D., Najoukas, M., Park, M., et al. (2005). The c-Src tyrosine kinase associates with the catalytic domain of ErbB-2: implications for ErbB-2 mediated signaling and transformation. Oncogene, 24, 7599–7607.
Muthuswamy, S. K., & Muller, W. J. (1995). Direct and specific interaction of c-Src with Neu is involved in signaling by the epidermal growth factor receptor. Oncogene, 11, 271–279.
Muthuswamy, S. K., Siegel, P. M., Dankort, D. L., Webster, M. A., & Muller, W. J. (1994). Mammary tumors expressing the neu proto-oncogene possess elevated c-Src tyrosine kinase activity. Molecular and Cellular Biology, 14, 735–743.
Acknowledgements
We gratefully acknowledge support by the Israel Science Foundation, founded by the Israel Academy of Sciences and Humanities; by the United States-Israel Binational Science Foundation; by the Israel Cancer Research Fund; by the US Army Research and Materiel Command; and by the Women’s Health Research Center at The Weizmann Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berman-Golan, D., Granot-Attas, S. & Elson, A. Protein tyrosine phosphatase epsilon and Neu-induced mammary tumorigenesis. Cancer Metastasis Rev 27, 193–203 (2008). https://doi.org/10.1007/s10555-008-9124-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-008-9124-0