Abstract
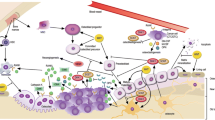
Patients with advanced breast cancer frequently develop metastasis to bone. Bone metastasis results in intractable pain and a high risk of fractures due to tumor-driven bone loss (osteolysis), which is caused by increased osteoclast activity. Osteolysis releases bone-bound growth factors including transforming growth factor beta (TGF-β). The widely accepted model of osteolytic bone metastasis in breast cancer is based on the hypothesis that the TGF-β released during osteolytic lesion development stimulates tumor cell parathyroid hormone related protein (PTHrP), causing stromal cells to secrete receptor activator of NFκB ligand (RANKL), thus increasing osteoclast differentiation. Elevated osteoclast numbers results in increased bone resorption, leading to more TGF-β being released from bone. This interaction between tumor cells and the bone microenvironment results in a vicious cycle of bone destruction and tumor growth. Bisphosphonates are commonly prescribed small molecule therapeutics that target tumor-driven osteoclastic activity in osteolytic breast cancers. In addition to bisphosphonate therapies, steroidal and non-steroidal antiestrogen and adjuvant therapies with aromatase inhibitors are additional small molecule therapies that may add to the arsenal for treatment of osteolytic breast cancer. This review focuses on a brief discussion of tumor-driven osteolysis and the effects of small molecule therapies in reducing osteolytic tumor progression.
Similar content being viewed by others
References
Martin, T. J., & Moseley, J. M. (2000). Mechanisms in the skeletal complications of breast cancer. Endocrine-Related Cancer, 7, 271–284.
Paget, S. (1889). The distribution of secondary growths in cancer of the breast. Lancet, 1, 571–573.
Guise, T. A., & Mundy, G. R. (1998). Cancer and bone. Endocrine Reviews, 19, 18–54.
Pederson, L., Winding, B., Foged, N. T., Spelsberg, T. C., & Oursler, M. J. (1999). Identification of breast cancer cell line-derived paracrine factors that stimulate osteoclast activity. Cancer Research, 59, 5849–5855.
Thomas, R. J., Guise, T. A., Yin, J. J., Elliott, J., Horwood, N. J., Martin, T. J., et al. (1999). Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology, 140, 4451–4458.
Lacroix, M., Siwek, B., Marie, P. J., & Body, J. J. (1998). Production and regulation of interleukin-11 by breast cancer cells. Cancer Letter, 127, 29–35.
Bendre, M., Gaddy, D., Nicholas, R. W., & Suva, L. J. (2003). Breast cancer metastasis to bone: It is not all about PTHrP. Clinical Orthopaedics and Related Research, S39–S45.
Tumber, A., Morgan, H. M., Meikle, M. C., & Hill, P. A. (2001). Human breast-cancer cells stimulate the fusion, migration and resorptive activity of osteoclasts in bone explants. International Journal of Cancer, 91, 665–672.
Morgan, H., Tumber, A., & Hill, P. A. (2004). Breast cancer cells induce osteoclast formation by stimulating host IL-11 production and downregulating granulocyte/macrophage colony-stimulating factor. International Journal of Cancer, 109, 653–660.
Mancino, A. T., Klimberg, V. S., Yamamoto, M., Manolagas, S. C., & Abe, E. (2001). Breast cancer increases osteoclastogenesis by secreting M-CSF and upregulating RANKL in stromal cells. Journal of Surgical Research, 100, 18–24.
Yin, J. J., Selander, K., Chirgwin, J. M., Dallas, M., Grubbs, B. G., Wieser, R., et al. (1999). TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. Journal of Clinical Investigation, 103, 197–206.
Sachdev, D., & Yee, D. (2001). The IGF system and breast cancer. Endocrine-Related Cancer, 8, 197–209.
Hauschka, P. V., Mavrakos, A. E., Iafrati, M. D., Doleman, S. E., & Klagsbrun, M. (1986). Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin–Sepharose. Journal of Biological Chemistry, 261, 12665–12674.
Hauschka, P. V., Chen, T. L., & Mavrakos, A. E. (1988). Polypeptide growth factors in bone matrix. Ciba Foundation Symposium, 136, 207–225.
Massague, J. (1998). TGF-beta signal transduction. Annual Review of Biochemistry, 67, 753–791.
Pluijm, G., Lowik, C., & Papapoulos, S. (2000). Tumour progression and angiogenesis in bone metastasis from breast cancer: New approaches to an old problem. Cancer Treatment Reviews, 26, 11–27.
Dougall, W. C., Glaccum, M., Charrier, K., Rohrbach, K., Brasel, K., De Smedt, T., et al. (1999). RANK is essential for osteoclast and lymph node development. Genes & Development, 13, 2412–2424.
Tanaka, S., Takahashi, N., Udagawa, N., Tamura, T., Akatsu, T., Stanley, E. R., et al. (1993). Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. Journal of Clinical Investigation, 91, 257–263.
Lacey, D. L., Timms, E., Tan, H. L., Kelley, M. J., Dunstan, C. R., Burgess, T., et al. (1998). Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell, 93, 165–176.
Bhatia, P., Sanders, M. M., & Hansen, M. F. (2005). Expression of receptor activator of nuclear factor-kappaB is inversely correlated with metastatic phenotype in breast carcinoma. Clinical Cancer Research, 11, 162–165.
Jones, D. H., Nakashima, T., Sanchez, O. H., Kozieradzki, I., Komarova, S. V., Sarosi, I., et al. (2006). Regulation of cancer cell migration and bone metastasis by RANKL. Nature, 440, 692–696.
Guise, T. A., Yin, J. J., Thomas, R. J., Dallas, M., Cui, Y., & Gillespie, M. T. (2002). Parathyroid hormone-related protein (PTHrP)–(1–139) isoform is efficiently secreted in vitro and enhances breast cancer metastasis to bone in vivo. Bone, 30, 670–676.
Saito, H., Tsunenari, T., Onuma, E., Sato, K., Ogata, E., & Yamada-Okabe, H. (2005). Humanized monoclonal antibody against parathyroid hormone-related protein suppresses osteolytic bone metastasis of human breast cancer cells derived from MDA-MB-231. Anticancer Research, 25, 3817–3823.
Wieser, R., Attisano, L., Wrana, J. L., & Massague, J. (1993). Signaling activity of transforming growth factor beta type II receptors lacking specific domains in the cytoplasmic region. Molecular and Cellular Biology, 13, 7239–7247.
Yang, Y. A., Dukhanina, O., Tang, B., Mamura, M., Letterio, J. J., MacGregor, J., et al. (2002). Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. Journal of Clinical Investigation, 109, 1607–1615.
Bendre, M. S., Montague, D. C., Peery, T., Akel, N. S., Gaddy, D., & Suva, L. J. (2003). Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone, 33, 28–37.
Burtis, W. J., Brady, T. G., Orloff, J. J., Ersbak, J. B., Warrell, R. P., Jr., Olson, B. R., et al. (1990). Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. New England Journal of Medicine, 322, 1106–1112.
Powell, G. J., Southby, J., Danks, J. A., Stillwell, R. G., Hayman, J. A., Henderson, M. A., et al. (1991). Localization of parathyroid hormone-related protein in breast cancer metastases: Increased incidence in bone compared with other sites. Cancer Research, 51, 3059–3061.
Henderson, M. A., Danks, J. A., Slavin, J. L., Byrnes, G. B., Choong, P. F., Spillane, J. B., et al. (2006). Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Research, 66, 2250–2256.
Fuller, K., Murphy, C., Kirstein, B., Fox, S. W., & Chambers, T. J. (2002). TNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology, 143, 1108–1118.
Oursler, M. J. (1994). Osteoclast synthesis and secretion and activation of latent transforming growth factor beta. Journal of Bone and Mineral Research, 9, 443–452.
Pfeilschifter, J., & Mundy, G. R. (1987). Modulation of type beta transforming growth factor activity in bone cultures by osteotropic hormones. Proceedings of the National Academy of Sciences of the United States of America, 84, 2024–2028.
Kozlow, W., & Guise, T. A. (2005). Breast cancer metastasis to bone: Mechanisms of osteolysis and implications for therapy. Journal of Mammary Gland Biology and Neoplasia, 10, 169–180.
Guise, T. A., Kozlow, W. M., Heras-Herzig, A., Padalecki, S. S., Yin, J. J., & Chirgwin, J. M. (2005). Molecular mechanisms of breast cancer metastases to bone. Clinical Breast Cancer, 5(Suppl), S46–S53.
Serra, R., & Crowley, M. R. (2003). TGF-beta in mammary gland development and breast cancer. Breast Disease, 18, 61–73.
Hildenbrand, R., Jansen, C., Wolf, G., Bohme, B., Berger, S., von Minckwitz, G., et al. (1998). Transforming growth factorbeta stimulates urokinase expression in tumorassociated macrophages of the breast. Laboratory Investigation, 78, 59–71.
Feng, X. H., & Derynck, R. (2005). Specificity and versatility in tgf-beta signaling through Smads. Annual Review of Cell and Developmental Biology, 21, 659–693.
Frey, R. S., & Mulder, K. M. (1997). TGFbeta regulation of mitogen-activated protein kinases in human breast cancer cells. Cancer Letter, 117, 41–50.
Kakonen, S. M., Selander, K. S., Chirgwin, J. M., Yin, J. J., Burns, S., Rankin, W. A., et al. (2002).Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. Journal of Biological Chemistry, 277, 24571–24578.
Lindemann, R. K., Ballschmieter, P., Nordheim, A., & Dittmer, J. (2001). Transforming growth factor beta regulates parathyroid hormone-related protein expression in MDA-MB-231 breast cancer cells through a novel Smad/Ets synergism. Journal of Biological Chemistry, 276, 46661–46670.
Deckers, M., van Dinther, M., Buijs, J., Que, I., Lowik, C., van der Pluijm, G., et al. (2006). The tumor suppressor Smad4 is required for transforming growth factor beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Research, 66, 2202–2209.
Serra, R., & Crowley, M. R. (2005). Mouse models of transforming growth factor beta impact in breast development and cancer. Endocrine-Related Cancer, 12, 749–760.
Mercer, R. R., Miyasaka, C., & Mastro, A. M. (2004). Metastatic breast cancer cells suppress osteoblast adhesion and differentiation. Clinical & Experimental Metastasis, 21, 427–435.
Mastro, A. M., Gay, C. V., Welch, D. R., Donahue, H. J., Jewell, J., Mercer, R., et al. (2004). Breast cancer cells induce osteoblast apoptosis: A possible contributor to bone degradation. Journal of Cellular Biochemistry, 91, 265–276.
Fleisch, H. (1991). Bisphosphonates. Pharmacology and use in the treatment of tumour-induced hypercalcaemic and metastatic bone disease. Drugs, 42, 919–944.
Brown, J. E., Neville-Webbe, H., & Coleman, R. E. (2004). The role of bisphosphonates in breast and prostate cancers. Endocrine-Related Cancer, 11, 207–224.
van der Pluijm, G., Vloedgraven, H., van Beek, E., van der Wee-Pals, L., Lowik, C., & Papapoulos, S. (1996). Bisphosphonates inhibit the adhesion of breast cancer cells to bone matrices in vitro. Journal of Clinical Investigation, 98, 698–705.
Boissier, S., Magnetto, S., Frappart, L., Cuzin, B., Ebetino, F. H., Delmas, P. D., et al. (1997). Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracellular matrices. Cancer Research, 57, 3890–3894.
Schmidt, A., Rutledge, S. J., Endo, N., Opas, E. E., Tanaka, H., Wesolowski, G., et al. (1996). Protein-tyrosine phosphatase activity regulates osteoclast formation and function: Inhibition by alendronate. Proceedings of the National Academy of Sciences of the United States of America, 93, 3068–3073.
Endo, N., Rutledge, S. J., Opas, E. E., Vogel, R., Rodan, G. A., & Schmidt, A. (1996). Human protein tyrosine phosphatase-sigma: Alternative splicing and inhibition by bisphosphonates. Journal of Bone and Mineral Research, 11, 535–543.
Skorey, K., Ly, H. D., Kelly, J., Hammond, M., Ramachandran, C., Huang, Z., et al. (1997). How does alendronate inhibit protein-tyrosine phosphatases? Journal of Biological Chemistry, 272, 22472–22480.
Bergstrom, J. D., Bostedor, R. G., Masarachia, P. J., Reszka, A. A., & Rodan, G. (2000). Alendronate is a specific, nanomolar inhibitor of farnesyl diphosphate synthase. Archives of Biochemistry and Biophysics, 373, 231–241.
Swanson, K. M., & Hohl, R. J. (2006). Anti-cancer therapy: Targeting the mevalonate pathway. Current Cancer Drug Targets, 6, 15–37.
Jagdev, S. P., Coleman, R. E., Shipman, C. M., Rostami, H. A., & Croucher, P. I. (2001). The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: Evidence for synergy with paclitaxel. British Journal of Cancer, 84, 1126–1134.
Yoneda, T., Michigami, T., Yi, B., Williams, P. J., Niewolna, M., & Hiraga, T. (2000). Actions of bisphosphonate on bone metastasis in animal models of breast carcinoma. Cancer, 88, 2979–2988.
Fromigue, O., Lagneaux, L., & Body, J. J. (2000). Bisphosphonates induce breast cancer cell death in vitro. Journal of Bone and Mineral Research, 15, 2211–2221.
Senaratne, S. G., Pirianov, G., Mansi, J. L., Arnett, T. R., & Colston, K. W. (2000). Bisphosphonates induce apoptosis in human breast cancer cell lines. British Journal of Cancer, 82, 1459–1468.
Neville-Webbe, H. L., Rostami-Hodjegan, A., Evans, C. A., Coleman, R. E., & Holen, I. (2005). Sequence- and schedule-dependent enhancement of zoledronic acid induced apoptosis by doxorubicin in breast and prostate cancer cells. International Journal of Cancer, 113, 364–371.
Journe, F., Chaboteaux, C., Magne, N., Duvillier, H., Laurent, G., & Body, J. J. (2006). Additive growth inhibitory effects of ibandronate and antiestrogens in estrogen receptor-positive breast cancer cell lines. Breast Cancer Research, 8, R2.
Boissier, S., Ferreras, M., Peyruchaud, O., Magnetto, S., Ebetino, F. H., Colombel, M., et al. (2000). Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Research, 60, 2949–2954.
Virtanen, S. S., Vaananen, H. K., Harkonen, P. L., & Lakkakorpi, P. T. (2002). Alendronate inhibits invasion of PC-3 prostate cancer cells by affecting the mevalonate pathway. Cancer Research, 62, 2708–2714.
Ferretti, G., Fabi, A., Carlini, P., Papaldo, P., Cordiali Fei, P., Di Cosimo, S., et al. (2005). Zoledronic-acid-induced circulating level modifications of angiogenic factors, metalloproteinases and proinflammatory cytokines in metastatic breast cancer patients. Oncology, 69, 35–43.
Hortobagyi, G. N., Theriault, R. L., Porter, L., Blayney, D., Lipton, A., Sinoff, C., et al. (1996). Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. New England Journal of Medicine, 335, 1785–1791.
Hortobagyi, G. N., & Piccart-Gebhart, M. J. (1996). Current management of advanced breast cancer. Seminars in Oncology, 23, 1–5.
Kanis, J. A. (1996). Rationale for the use of bisphosphonates in breast cancer. Acta Oncologica, 35(Suppl 5), 61–67.
Theriault, R. L., Lipton, A., Hortobagyi, G. N., Leff, R., Gluck, S., Stewart, J. F., et al. (1999). Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: A randomized, placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. Journal of Clinical Oncology, 17, 846–854.
Hurst, M., & Noble, S. (1999). Clodronate: A review of its use in breast cancer. Drugs and Aging, 15, 143–167.
Diel, I. J., Solomayer, E. F., Costa, S. D., Gollan, C., Goerner, R., Wallwiener, D., et al. (1998). Reduction in new metastases in breast cancer with adjuvant clodronate treatment. New English Journal of Medicine, 339, 357–363.
Kage, K., Nagahama, T., Sekine, I., Maruyama, M., & Ogata, E. (1997). A remarkable improvement of clinical manifestations in a breast cancer patient with widespread bone metastases after administration of pamidronate. Internal Medicine, 36, 926–930.
Luckman, S. P., Hughes, D. E., Coxon, F. P., Graham, R., Russell, G., & Rogers, M. J. (1998). Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. Journal of Bone and Mineral Research, 13, 581–589.
Delmas, P. D., Balena, R., Confravreux, E., Hardouin, C., Hardy, P., & Bremond, A. (1997). Bisphosphonate risedronate prevents bone loss in women with artificial menopause due to chemotherapy of breast cancer: A double-blind, placebo-controlled study. Journal of Clinical Oncology, 15, 955–962.
Body, J. J., Diel, I. J., Lichinitser, M. R., Kreuser, E. D., Dornoff, W., Gorbunova, V. A., et al. (2003). Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Annals of Oncology, 14, 1399–1405.
Body, J. J., Diel, I. J., Bell, R., Pecherstorfer, M., Lichinitser, M. R., Lazarev, A. F., et al. (2004). Oral ibandronate improves bone pain and preserves quality of life in patients with skeletal metastases due to breast cancer. Pain, 111, 306–312.
Hiraga, T., Williams, P. J., Mundy, G. R., & Yoneda, T. (2001). The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases. Cancer Research, 61, 4418–4424.
Hiraga, T., Williams, P. J., Ueda, A., Tamura, D., & Yoneda, T. (2004). Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clinical Cancer Research, 10, 4559–4567.
Ibrahim, A., Scher, N., Williams, G., Sridhara, R., Li, N., Chen, G., et al. (2003). Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clinical Cancer Research, 9, 2394–2399.
Wardley, A., Davidson, N., Barrett-Lee, P., Hong, A., Mansi, J., Dodwell, D., et al. (2005). Zoledronic acid significantly improves pain scores and quality of life in breast cancer patients with bone metastases: A randomised, crossover study of community vs hospital bisphosphonate administration. British Journal of Cancer, 92, 1869–1876.
Wood, J., Bonjean, K., Ruetz, S., Bellahcene, A., Devy, L., Foidart, J. M., et al. (2002). Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. Journal of Pharmacology and Experimental Therapeutics, 302, 1055–1061.
Senaratne, S. G., Mansi, J. L., & Colston, K. W. (2002). The bisphosphonate zoledronic acid impairs Ras membrane [correction of impairs membrane] localisation and induces cytochrome c release in breast cancer cells. British Journal of Cancer, 86, 1479–1486.
Hillner, B. E., Ingle, J. N., Berenson, J. R., Janjan, N. A., Albain, K. S., Lipton, A., et al. (2000). American Society of Clinical Oncology guideline on the role of bisphosphonates in breast cancer. American Society of Clinical Oncology Bisphosphonates Expert Panel. Journal of Clinical Oncology, 18, 1378–1391.
Santen, R. J., & Harvey, H. A. (1999). Use of aromatase inhibitors in breast carcinoma. Endocrine-Related Cancer, 6, 75–92.
Jordan, V. C. (2001). Selective estrogen receptor modulation: A personal perspective. Cancer Research, 61, 5683–5687.
Early Breast Cancer Trialists’ Collaborative Group (1998).Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet, 351, 1451–1467.
Jordan, V. C., & Koerner, S. (1975). Tamoxifen (ICI 46,474) and the human carcinoma 8S oestrogen receptor. European Journal of Cancer, 11, 205–206.
Jordan, V. C., & Prestwich, G. (1977). Binding of [3H]tamoxifen in rat uterine cytosols: A comparison of swinging bucket and vertical tube rotor sucrose density gradient analysis. Molecular and Cellular Endocrinology, 8, 179–188.
Osborne, C. K. (1998). Tamoxifen in the treatment of breast cancer. New England Journal of Medicine, 339, 1609–1618.
Cole, M. P., Jones, C. T., & Todd, I. D. (1971). A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. British Journal of Cancer, 25, 270–275.
Ward, H. W. (1973). Anti-oestrogen therapy for breast cancer: A trial of tamoxifen at two dose levels. British Medical Journal, 1, 13–14.
Love, R. R., Wiebe, D. A., Newcomb, P. A., Cameron, L., Leventhal, H., Jordan, V. C., et al. (1991). Effects of tamoxifen on cardiovascular risk factors in postmenopausal women. Annals of Internal Medicine, 115, 860–864.
Love, R. R., Mazess, R. B., Barden, H. S., Epstein, S., Newcomb, P. A., Jordan, V. C., et al. (1992). Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. New England Journal of Medicine, 326, 852–856.
Litherland, S., & Jackson, I. M. (1988). Antioestrogens in the management of hormone-dependent cancer. Cancer Treatment Reviews, 15, 183–194.
Sporn, M. B., Dowsett, S. A., Mershon, J., & Bryant, H. U. (2004). Role of raloxifene in breast cancer prevention in postmenopausal women: Clinical evidence and potential mechanisms of action. Clinical Therapeutics, 26, 830–840.
Lamb, C. A., Helguero, L. A., Fabris, V., Lucas, C., Molinolo, A. A., & Lanari, C. (2003). Differential effects of raloxifene, tamoxifen and fulvestrant on a murine mammary carcinoma. Breast Cancer Research and Treatment, 79, 25–35.
Taranta, A., Brama, M., Teti, A., De luca, V., Scandurra, R., Spera, G., et al. (2002). The selective estrogen receptor modulator raloxifene regulates osteoclast and osteoblast activity in vitro. Bone, 30, 368–376.
Jordan, V. C. (1998). Designer estrogens. Scientific American, 279, 60–67.
Gradishar, W., Glusman, J., Lu, Y., Vogel, C., Cohen, F. J., & Sledge, G. W., Jr. (2000). Effects of high dose raloxifene in selected patients with advanced breast carcinoma. Cancer, 88, 2047–2053.
Vogel, V. G., Costantino, J. P., Wickerham, D. L., Cronin, W. M., Cecchini, R. S., Atkins, J. N., et al. (2006). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA, 295, 2727–2741.
Sato, M., Turner, C. H., Wang, T., Adrian, M. D., Rowley, E., & Bryant, H. U. (1998). LY353381.HCl: A novel raloxifene analog with improved SERM potency and efficacy in vivo. Journal of Pharmacology and Experimental Therapeutics, 287, 1–7.
Fabian, C. J., Kimler, B. F., Anderson, J., Tawfik, O. W., Mayo, M. S., Burak, W. E., Jr., et al. (2004). Breast cancer chemoprevention phase I evaluation of biomarker modulation by arzoxifene, a third generation selective estrogen receptor modulator. Clinical Cancer Research, 10, 5403–5417.
Grese, T. A., Pennington, L. D., Sluka, J. P., Adrian, M. D., Cole, H. W., Fuson, T. R., et al. (1998). Synthesis and pharmacology of conformationally restricted raloxifene analogues: Highly potent selective estrogen receptor modulators. Journal of Medicinal Chemistry, 41, 1272–1283.
Kangas, L. (1990). Biochemical and pharmacological effects of toremifene metabolites. Cancer Chemotherapy and Pharmacology, 27, 8–12.
Ke, H. Z., Paralkar, V. M., Grasser, W. A., Crawford, D. T., Qi, H., Simmons, H. A., et al. (1998). Effects of CP-336,156, a new, nonsteroidal estrogen agonist/antagonist, on bone, serum cholesterol, uterus and body composition in rat models. Endocrinology, 139, 2068–2076.
McClung, M. R., Siris, E., Cummings, S., Bolognese, M., Ettinger, M., Moffett, A., et al. (2006). Prevention of bone loss in postmenopausal women treated with lasofoxifene compared with raloxifene. Menopause, 13, 377–386.
Hayes, D. F., Van Zyl, J. A., Hacking, A., Goedhals, L., Bezwoda, W. R., Mailliard, J. A., et al. (1995). Randomized comparison of tamoxifen and two separate doses of toremifene in postmenopausal patients with metastatic breast cancer. Journal of Clinical Oncology, 13, 2556–2566.
Addo, S., Yates, R. A., & Laight, A. (2002). A phase I trial to assess the pharmacology of the new oestrogen receptor antagonist fulvestrant on the endometrium in healthy postmenopausal volunteers. British Journal of Cancer, 87, 1354–1359.
Hu, X. F., Veroni, M., De Luise, M., Wakeling, A., Sutherland, R., Watts, C. K., et al. (1993). Circumvention of tamoxifen resistance by the pure anti-estrogen ICI 182,780. International Journal of Cancer, 55, 873–876.
Smith, I. E., & Dowsett, M. (2003). Aromatase inhibitors in breast cancer. New England Journal of Medicine, 348, 2431–2442.
Mouridsen, H., & Gershanovich, M. (2003). The role of aromatase inhibitors in the treatment of metastatic breast cancer. Seminars in Oncology, 30, 33–45.
Simpson, E. R. (2003). Sources of estrogen and their importance. Journal of Steroid Biochemistry and Molecular Biology, 86, 225–230.
Bulun, S. E., Mahendroo, M. S., & Simpson, E. R. (1994). Aromatase gene expression in adipose tissue: Relationship to breast cancer. Journal of Steroid Biochemistry and Molecular Biology, 49, 319–326.
Bulun, S. E., & Simpson, E. R. (1994). Regulation of aromatase expression in human tissues. Breast Cancer Research and Treatment, 30, 19–29.
Baum, M., Buzdar, A., Cuzick, J., Forbes, J., Houghton, J., Howell, A., et al. (2003). Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: Results of the ATAC (Arimidex, Tamoxifen alone or in combination) trial efficacy and safety update analyses. Cancer, 98, 1802–1810.
Howell, A., Robertson, J. F., & Vergote, I. (2003). A review of the efficacy of anastrozole in postmenopausal women with advanced breast cancer with visceral metastases. Breast Cancer Research and Treatment, 82, 215–222.
Baum, M., & Buzdar, A. (2003). The current status of aromatase inhibitors in the management of breast cancer. Surgical Clinics of North America, 83, 973–994.
Goss, P. E., Ingle, J. N., Martino, S., Robert, N. J., Muss, H. B., Piccart, M. J., et al. (2003). A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. New England Journal of Medicine, 349, 1793–1802.
Goss, P. E., Ingle, J. N., Martino, S., Robert, N. J., Muss, H. B., Piccart, M. J., et al. (2005). Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: Updated findings from NCIC CTG MA.17. Journal of the National Cancer Institute, 97, 1262–1271.
Smith, I. E. (2003). Letrozole versus tamoxifen in the treatment of advanced breast cancer and as neoadjuvant therapy. Journal of Steroid Biochemistry and Molecular Biology, 86, 289–293.
Mouridsen, H., Gershanovich, M., Sun, Y., Perez-Carrion, R., Boni, C., Monnier, A., et al. (2001). Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: Results of a phase III study of the International Letrozole Breast Cancer Group. Journal of Clinical Oncology, 19, 2596–2606.
Mouridsen, H., Gershanovich, M., Sun, Y., Perez-Carrion, R., Boni, C., Monnier, A., et al. (2003). Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: Analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. Journal of Clinical Oncology, 21, 2101–2109.
Thurlimann, B., Keshaviah, A., Coates, A. S., Mouridsen, H., Mauriac, L., Forbes, J. F., et al. (2005). A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. New England Journal of Medicine, 353, 2747–2757.
Mincey, B. A., Duh, M. S., Thomas, S. K., Moyneur, E., Marynchencko, M., Boyce, S. P., et al. (2006). Risk of cancer treatment-associated bone loss and fractures among women with breast cancer receiving aromatase inhibitors. Clinical Breast Cancer, 7, 127–132.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cicek, M., Oursler, M.J. Breast cancer bone metastasis and current small therapeutics. Cancer Metastasis Rev 25, 635–644 (2006). https://doi.org/10.1007/s10555-006-9035-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-006-9035-x