Abstract
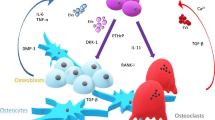
Breast cancer cells preferentially spread to bone. Bone metastases are currently incurable and therefore better treatments need to be developed. Metastasis is an inefficient, multi-step process. Specific aspects of both breast cancer cells and the bone microenvironment contribute to the development of bone metastases. Breast cancers express chemokine receptors, integrins, cadherins, and bone-resorbing and bone-forming factors that contribute to the successful and preferential spread of tumor to bone. Bone is rich in growth factors and cell types that make it a hospitable environment for breast cancer growth. Once breast cancer cells enter the bone, a highly complex vicious cycle develops, in which breast cancer cells secrete factors that act on bone cells and other cells within the bone (stem cells, T cells, platelets, adipocytes, fibroblasts, and endothelial cells), causing them to secrete factors that act on adjacent cancer cells. The steps in the metastatic cascade and the vicious cycle within bone offer unique targets for adjuvant treatments to treat and cure bone metastases.
Similar content being viewed by others
References
Coleman, R. E. (1997). Skeletal complications of malignancy. Cancer, 80, 1588–1594.
Kozlow, W., & Guise, T. A. (2005). Breast cancer metastasis to bone: Mechanisms of osteolysis and implications for therapy. Journal of Mammary Gland Biology and Neoplasia, 10, 169–180.
Boyce, B. F., Yoneda, T., & Guise, T. A. (1999). Factors regulating the growth of metastatic cancer in bone. Endocrine Related Cancer, 6, 333–347.
Clines, G. A., & Guise, T. A. (2005). Hypercalcaemia of malignancy and basic research on mechanisms responsible for osteolytic and osteoblastic metastasis to bone. Endocrine Related Cancer, 12, 549–583.
Fidler, I. J. (2003). The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nature Reviews. Cancer, 3, 453–458.
Paget, S. (1989). The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Reviews, 8, 98–101.
Walther, H. E. (1948). Krebsmatastasen. Switzerland: Bens Schwabe Verlag.
Cifuentes, N., & Pickren, J. W. (1979). Metastases from carcinoma of mammary gland: An autopsy study. Journal of Surgical Oncology, 11, 193–205.
Weiss, L. (1992). Comments on hematogenous metastatic patterns in humans as revealed by autopsy. Clinical & Experimental Metastasis, 10, 191–199.
Coleman, R. E., & Rubens, R. D. (1987). The clinical course of bone metastases from breast cancer. British Journal of Cancer, 55, 61–66.
Kang, Y., Siegel, P. M., Shu, W., Drobnjak, M., Kakonen, S. M., Cordon-Cardo C., et al. (2003). A multigenic program mediating breast cancer metastasis to bone. Cancer Cell, 3, 537–549.
van der Pluijm, G., Sijmons, B., Vloedgraven, H., Deckers, M., Papapoulos, S., & Lowik, C. (2001). Monitoring metastatic behavior of human tumor cells in mice with species-specific polymerase chain reaction: Elevated expression of angiogenesis and bone resorption stimulators by breast cancer in bone metastases. Journal of Bone and Mineral Research, 16, 1077–1091.
Luker, K. E., & Luker, G. D. (2006). Functions of CXCL12 and CXCR4 ifsn breast cancer. Cancer Letter, 238, 30–41.
Shim, H., Lau, S. K., Devi, S., Yoon, Y., Cho, H. T., & Liang, Z. (2006). Lower expression of CXCR4 in lymph node metastases than in primary breast cancers: Potential regulation by ligand-dependent degradation and HIF-1alpha. Biochemical and Biophysical Research Communications, 346, 252–258.
Salvucci, O., Bouchard, A., Baccarelli, A., Deschenes, J., Sauter, G., Simon, R., et al. (2006). The role of CXCR4 receptor expression in breast cancer: A large tissue microarray study. Breast Cancer Research and Treatment, 97, 275–283.
Sloan, E. K., & Anderson, R. L. (2002). Genes involved in breast cancer metastasis to bone. Cellular and Molecular Life Sciences, 59, 1491–1502.
Muller, A., Homey, B., Soto, H., Ge, N., Catron, D., Buchanan, M. E., et al. (2001). Involvement of chemokine receptors in breast cancer metastasis. Nature, 410, 50–56.
Sun, Y. X., Schneider, A., Jung, Y., Wang, J., Dai, J., Wang, J., et al. (2005). Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. Journal of Bone and Mineral Research, 20, 318–329.
Liang, Z., Wu, T., Lou, H., Yu, X., Taichman, R. S., Lau, S. K., et al. (2004). Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Research, 64, 4302–4308.
Liang, Z., Yoon, Y., Votaw, J., Goodman, M. M., Williams, L., & Shim, H. (2005). Silencing of CXCR4 blocks breast cancer metastasis. Cancer Research, 65, 967–971.
Leonard, J. T., & Roy, K. (2006). The HIV entry inhibitors revisited. Current Medicinal Chemistry, 13, 911–934.
Harms, J. F., Welch, D. R., Samant, R. S., Shevde, L. A., Miele, M. E., Babu, G. R., et al. (2004). A small molecule antagonist of the alpha(v)beta3 integrin suppresses MDA-MB-435 skeletal metastasis. Clinical & Experimental Metastasis, 21, 119–128.
Cacciari, B., & Spalluto, G. (2005). Non peptidic alphavbeta3 antagonists: Recent developments. Current Medicinal Chemistry, 12, 51–70.
Liapis, H., Flath, A., & Kitazawa, S. (1996). Integrin alpha V beta 3 expression by bone-residing breast cancer metastases. Diagnostic Molecular Pathology, 5, 127–135.
Felding-Habermann, B., O’Toole, T. E., Smith, J. W., Fransvea, E., Ruggeri, Z. M., Ginsberg, M. H., et al. (2001). Integrin activation controls metastasis in human breast cancer. Proceedings of the National Academy of Sciences of the United States of America, 98, 1853–1858.
Sloan, E. K., Pouliot, N., Stanley, K. L., Chia, J., Moseley, J. M., Hards, D. K., et al. (2006). Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Research, 8, R20.
Beekman, K. W., Colevas, A. D., Cooney, K., Dipaola, R., Dunn, R. L., Gross, M., et al. (2006). Phase II evaluations of cilengitide in asymptomatic patients with androgen-independent prostate cancer: Scientific rationale and study design. Clinical Genitourinary Cancer, 4, 299–302.
Yoneda, T., & Hiraga, T. (2005). Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochemical and Biophysical Research Communications, 328, 679–687.
Mbalaviele, G., Dunstan, C. R., Sasaki, A., Williams, P. J., Mundy, G. R., & Yoneda, T. (1996). E-cadherin expression in human breast cancer cells suppresses the development of osteolytic bone metastases in an experimental metastasis model. Cancer Research, 56, 4063–4070.
Hazan, R. B., Phillips, G. R., Qiao, R. F., Norton, L., & Aaronson, S. A. (2000). Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. Journal of Cell Biology, 148, 779–790.
Bachmeier, B. E., Nerlich, A. G., Lichtinghagen, R., & Sommerhoff, C. P. (2001). Matrix metalloproteinases (MMPs) in breast cancer cell lines of different tumorigenicity. Anticancer Research, 21, 3821–3828.
Nakopoulou, L., Tsirmpa, I., Alexandrou, P., Louvrou, A., Ampela, C., Markaki, S., et al. (2003). MMP-2 protein in invasive breast cancer and the impact of MMP-2/TIMP-2 phenotype on overall survival. Breast Cancer Research and Treatment, 77, 145–155.
Zhao, W., Byrne, M. H., Boyce., B. F., & Krane, S. M. (1999). Bone resorption induced by parathyroid hormone is strikingly diminished in collagenase-resistant mutant mice. Journal of Clinical Investigation, 103, 517–524.
Coussens, L. M., Fingleton, B., & Matrisian, L. M. (2002). Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science, 295, 2387–2392.
Overall, C. M., & Lopez-Otin, C. (2002). Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nature Reviews. Cancer, 2, 657–672.
Guise, T. A., Kozlow, W. M., Heras-Herzig, A., Padalecki, S. S., Yin, J. J., & Chirgwin, J. M. (2005). Molecular mechanisms of breast cancer metastases to bone. Clinical Breast Cancer, 5 (Suppl), S46–53.
Abou-Samra, A. B., Juppner, H., Force, T., Freeman, M. W., Kong, X. F., Schipani, E., et al. (1992). Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: A single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proceedings of the National Academy of Sciences of the United States of America, 89, 2732–2736.
Pierroz, D. D., Bouxsein, M. L., Rizzoli, R., & Ferrari, S. L. (2006). Combined treatment with a beta-blocker and intermittent PTH improves bone mass and microarchitecture in ovariectomized mice. Bone, 39, 260–267.
Guise, T. A., Yin, J. J., Taylor, S. D., Kumagai, Y., Dallas, M., Boyce, B. F., et al. (1996). Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. Journal of Clinical Investigation, 98, 1544–1549.
Thomas, R. J., Guise, T. A., Yin, J. J., Elliott, J., Horwood, N. J., Martin, T. J., et al. (1999). Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology, 140, 4451–4458.
Henderson, M., Danks, J., Moseley, J., Slavin, J., Harris, T., McKinlay, M., et al. (2001). Parathyroid hormone-related protein production by breast cancers, improved survival, and reduced bone metastases. Journal of the National Cancer Institute, 93, 234–237.
de la Mata, J., Uy, H. L., Guise, T. A., Story, B., Boyce, B. F., Mundy, G. R., et al. (1995). Interleukin-6 enhances hypercalcemia and bone resorption mediated by parathyroid hormone-related protein in vivo. Journal of Clinical Investigation, 95, 2846–2852.
Kakonen, S., Kang, Y., Carreon, M., Niewolna, M., Kakonen, R., Chirgwin, J., et al. (2002). Breast cancer cell lines selected from bone metastases have greater metastatic capacity and express increased vascular endothelial growth factor (VEGF), interleukin-11 (IL-11), and parathyroild hormone-related protein (PTHrP) “abstract”. Journal of Bone and Mineral Research, 17, M060.
Bendre, M. S., Margulies, A. G., Walser, B., Akel, N. S., Bhattacharrya, S., Skinner, R. A., et al. (2005). Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Research, 65, 11001–11009.
Yin, J. J., Mohammad, K. S., Kakonen, S. M., Harris, S., Wu-Wong, J. R., Wessale, J. L., et al. (2003). A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proceedings of the National Academy of Sciences of the United States of America, 100, 10954–10959.
Chirgwin, J. M., Mohammad, K. S., & Guise, T. A. (2004). Tumor–bone cellular interactions in skeletal metastases. Journal of Musculoskeletal & Neuronal Interactions, 4, 308–318.
Semenza, G. L. (2003). Targeting HIF-1 for cancer therapy. Nature Reviews. Cancer, 3, 721–732.
Yin, J. J., Selander, K., Chirgwin, J. M., Dallas, M., Grubbs, B. G., Wieser, R., et al. (1999). TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. Journal of Clinical Investigation, 103, 197–206.
van ’t Veer, L. J., Dai, H., van de Vijver, M. J., He, Y. D., Hart, A. A., Mao, M., et al. (2002). Gene expression profiling predicts clinical outcome of breast cancer. Nature, 415, 530–536, 2002.
Yoneda, T., Williams, P. J., Hiraga, T., Niewolna, M., & Nishimura, R. (2001). A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. Journal of Bone and Mineral Research, 16, 1486–1495.
Myoui, A., Nishimura, R., Williams, P. J., Hiraga, T., Tamura, D., Michigami, T., et al. (2003). C-SRC tyrosine kinase activity is associated with tumor colonization in bone and lung in an animal model of human breast cancer metastasis. Cancer Research, 63, 5028–5033.
Rucci, N., Recchia, I., Angelucci, A., Alamanou, M., Del Fattore, A., Fortunati, D., et al. (2006). Inhibition of protein kinase c-Src reduces the incidence of breast cancer metastases and increases survival in mice: Implications for therapy. Journal of Pharmacology and Experimental Therapeutics, 318, 161–172.
Shakespeare, W. C., Metcalf, C. A., 3rd, Wang, Y., Sundaramoorthi, R., Keenan, T., Weigele, M., et al. (2003). Novel bone-targeted Src tyrosine kinase inhibitor drug discovery. Current Opinion in Drug Discovery and Development, 6, 729–741.
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2002). Molecular Biology of THE CELL: (4th ed., pp. 1259–1312). New York: Garland Science.
Hauschka, P. V., Mavrakos, A. E., Iafrati, M. D., Doleman, S. E., & Klagsbrun, M. (1986). Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. Journal of Biological Chemistry, 261, 12665–12674.
Parisi, M. S., Gazzerro, E., Rydziel, S., & Canalis, E. (2006). Expression and regulation of CCN genes in murine osteoblasts. Bone, 38, 671–677.
Simonet, W. S., Lacey, D. L., Dunstan, C. R., Kelley, M., Chang, M. S., Luthy, R., et al. (1997). Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell, 89, 309–319.
Cornish, J., Naot, D., & Reid, I. R. (2003). Adrenomedullin—A regulator of bone formation. Regulatory Peptides, 112, 79–86.
Rifas, L., Halstead, L. R., Peck, W. A., Avioli, L. V., & Welgus, H. G. (1989). Human osteoblasts in vitro secrete tissue inhibitor of metalloproteinases and gelatinase but not interstitial collagenase as major cellular products. Journal of Clinical Investigation, 84, 686–694.
Harada, S., Nagy, J. A., Sullivan, K. A., Thomas, K. A., Endo, N., Rodan, G. A., et al. (1994). Induction of vascular endothelial growth factor expression by prostaglandin E2 and E1 in osteoblasts. Journal of Clinical Investigation, 93, 2490–2496.
Felix, R., Halasy-Nagy, J., Wetterwald, A., Cecchini, M. G., Fleisch, H., & Hofstetter, W. (1996). Synthesis of membrane- and matrix-bound colony-stimulating factor-1 by cultured osteoblasts. Journal of Cellular Physiology, 166, 311–322.
Schmidt, C., Steinbach, G., Decking, R., Claes, L. E., Ignatius, A. A. (2003). IL-6 and PGE2 release by human osteoblasts on implant materials. Biomaterials, 24, 4191–4196.
Taichman, R., Reilly, M., Verma, R., Ehrenman, K., & Emerson, S. (2001). Hepatocyte growth factor is secreted by osteoblasts and cooperatively permits the survival of haematopoietic progenitors. British Journal of Haematology, 112, 438–448.
Kostenuik, P. J., & Shalhoub, V. (2001). Osteoprotegerin: A physiological and pharmacological inhibitor of bone resorption. Current Pharmaceutical Design, 7, 613–635.
Yoshiko, Y., Son, A., Maeda, S., Igarashi, A., Takano, S., Hu, J., et al. (1999). Evidence for stanniocalcin gene expression in mammalian bone. Endocrinology, 140, 1869–1874.
Dallas, S. L., Rosser, J. L., Mundy, G. R., & Bonewald, L. F. (2002). Proteolysis of latent transforming growth factor-beta (TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix. Journal of Biological Chemistry, 277, 21352–21360.
Wakefield, L. M., & Roberts, A. B. (2002). TGF-beta signaling: Positive and negative effects on tumorigenesis. Current Opinion in Genetics and Development, 12, 22–29.
Kakonen, S. M., Selander, K. S., Chirgwin, J. M., Yin, J. J., Burns, S., Rankin, W. A., et al. (2002). Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. Journal of Biological Chemistry, 277, 24571–24578.
Muraoka, R. S., Dumont, N., Ritter, C. A., Dugger, T. C., Brantley, D. M., Chen, J., et al. (2002). Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. Journal of Clinical Investigation, 109, 1551–1559.
Yang, Y. A., Dukhanina, O., Tang, B., Mamura, M., Letterio, J. J., MacGregor, J., et al. (2002). Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. Journal of Clinical Investigation, 109, 1607–1615.
Bandyopadhyay, A., Agyin, J. K., Wang, L., Tang, Y., Lei, X., Story, B. M., et al. (2006). Inhibition of pulmonary and skeletal metastasis by a transforming growth factor-beta type I receptor kinase inhibitor. Cancer Research, 66, 6714–6721.
Ge, R., Rajeev, V., Ray, P., Lattime, E., Rittling, S., Medicherla, S., et al. (2006). Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-beta type I receptor kinase in vivo. Clinical Cancer Research, 12, 4315–4330.
Mitsiades, C. S., Mitsiades N. S., McMullan, C. J., Poulaki, V., Shringarpure, R., Akiyama, M., et al. (2004). Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell, 5, 221–230.
Goya, M., Miyamoto, S., Nagai, K., Ohki, Y., Nakamura, K., Shitara, K., et al. (2004). Growth inhibition of human prostate cancer cells in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice by a ligand-specific antibody to human insulin-like growth factors. Cancer Research, 64, 6252–6258.
van Golen, C. M., Schwab, T. S., Kim, B., Soules, M. E., Su Oh, S., Fung, K., et al. (2006). Insulin-like growth factor-I receptor expression regulates neuroblastoma metastasis to bone. Cancer Research, 66, 6570–6578.
Rubin, J., Fan, X., Rahnert, J., Sen, B., Hsieh, C. L., Murphy, T. C., et al. (2006). IGF-I secretion by prostate carcinoma cells does not alter tumor-bone cell interactions in vitro or in vivo. Prostate, 66, 789–800.
Wozney, J. M. (1992). The bone morphogenetic protein family and osteogenesis. Molecular Reproduction and Development, 32, 160–167.
Arnold, S. F., Tims, E., & McGrath, B. E. (1999). Identification of bone morphogenetic proteins and their receptors in human breast cancer cell lines: Importance of BMP2. Cytokine, 11, 1031–1037.
Pouliot, F., Blais, A., & Labrie, C. (2003). Overexpression of a dominant negative type II bone morphogenetic protein receptor inhibits the growth of human breast cancer cells. Cancer Research, 63, 277–281.
Ghosh-Choudhury, N., Ghosh-Choudhury, G., Celeste, A., Ghosh, P. M., Moyer, M., Abboud, S. L., et al. (2000). Bone morphogenetic protein-2 induces cyclin kinase inhibitor p21 and hypophosphorylation of retinoblastoma protein in estradiol-treated MCF-7 human breast cancer cells. Biochimica et Biophysica Acta, 1497, 186–196.
Helms, M. W., Packeisen, J., August, C., Schittek, B., Boecker, W., Brandt, B. H., et al. (2005). First evidence supporting a potential role for the BMP/SMAD pathway in the progression of oestrogen receptor-positive breast cancer. Journal of Pathology, 206, 366–376.
Clement, J. H., Raida, M., Sanger, J., Bicknell, R., Liu, J., Naumann, A., et al. (2005). Bone morphogenetic protein 2 (BMP-2) induces in vitro invasion and in vivo hormone independent growth of breast carcinoma cells. International Journal of Oncology, 27, 401–407.
Valta, M. P., Hentunen, T., Qu, Q., Valve, E. M., Harjula, A., Seppanen, J. A., et al. (2006). Regulation of osteoblast differentiation: A novel function for fibroblast growth factor 8. Endocrinology, 147, 2171–2182.
Ornitz, D. M. (2005). FGF signaling in the developing endochondral skeleton. Cytokine & Growth Factor Reviews, 16, 205–213.
Moursi, A. M., Winnard, P. L., Winnard, A. V., Rubenstrunk, J. M., & Mooney, M. P. (2002). Fibroblast growth factor 2 induces increased calvarial osteoblast proliferation and cranial suture fusion. Cleft Palate Craniofacial Journal, 39, 487–496.
Chikazu, D., Katagiri, M., Ogasawara, T., Ogata, N., Shimoaka, T., Takato, T., et al. (2001). Regulation of osteoclast differentiation by fibroblast growth factor 2: Stimulation of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor expression in osteoblasts and inhibition of macrophage colony-stimulating factor function in osteoclast precursors. Journal of Bone and Mineral Research, 16, 2074–2081.
Yoshimura, N., Sano, H., Hashiramoto, A., Yamada, R., Nakajima, H., Kondo, M., et al. (1998). The expression and localization of fibroblast growth factor-1 (FGF-1) and FGF receptor-1 (FGFR-1) in human breast cancer. Clinical Immunology and Immunopathology, 89, 28–34.
Okunieff, P., Fenton, B. M., Zhang, L., Kern, F. G., Wu, T., Greg, J. R., et al. (2003). Fibroblast growth factors (FGFS) increase breast tumor growth rate, metastases, blood flow, and oxygenation without significant change in vascular density. Advances in Experimental in Medicine and Biology, 530, 593–601.
Liu, J. F., Crepin, M., Liu, J. M., Barritault, D., & Ledoux, D.(2002). FGF-2 and TPA induce matrix metalloproteinase-9 secretion in MCF-7 cells through PKC activation of the Ras/ERK pathway. Biochemical and Biophysical Research Communications, 293, 1174–1182.
Yi, B., Williams, P. J., Niewolna, M., Wang, Y., & Yoneda, T. (2002). Tumor-derived platelet-derived growth factor-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Research, 62, 917–923.
Franchimont, N., & Canalis, E. (1995). Platelet-derived growth factor stimulates the synthesis of interleukin-6 in cells of the osteoblast lineage. Endocrinology, 136, 5469–5475.
Seymour, L., Dajee, D., & Bezwoda, W. R. (1993). Tissue platelet derived-growth factor (PDGF) predicts for shortened survival and treatment failure in advanced breast cancer. Breast Cancer Research and Treatment, 26, 247–252.
Seymour, L., & Bezwoda, W. R. (1994). Positive immunostaining for platelet derived growth factor (PDGF) is an adverse prognostic factor in patients with advanced breast cancer. Breast Cancer Research and Treatment, 32, 229–233.
Lev, D. C., Kim, S. J., Onn, A., Stone, V., Nam, D. H., Yazici, S., et al. (2005). Inhibition of platelet-derived growth factor receptor signaling restricts the growth of human breast cancer in the bone of nude mice. Clinical Cancer Research, 11, 306–314.
Silver, I. A., Murrills, R. J., & Etherington, D. J. (1988). Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Experimental Cell Research, 175, 266–276.
Sanders, J. L., Chattopadhyay, N., Kifor, O., Yamaguchi, T., Butters, R. R., & Brown, E. M. (2000). Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology, 141, 4357–4364.
Southby, J., Kissin, M. W., Danks, J. A., Hayman, J. A., Moseley, J. M., Henderson, M. A., et al. (1990). Immunohistochemical localization of parathyroid hormone-related protein in human breast cancer. Cancer Research, 50, 7710–7716.
Powell, G. J., Southby, J., Danks, J. A., Stillwell, R. G., Hayman, J. A., Henderson, M. A., et al. (1991). Localization of parathyroid hormone-related protein in breast cancer metastases: Increased incidence in bone compared with other sites. Cancer Research, 51, 3059–3061.
Vargas, S. J., Gillespie, M. T., Powell, G. J., Southby, J., Danks, J. A., Moseley, J. M., et al. (1992). Localization of parathyroid hormone-related protein mRNA expression in breast cancer and metastatic lesions by in situ hybridization. Journal of Bone and Mineral Research, 7, 971–979.
Nemeth, E. F. (2002). The search for calcium receptor antagonists (calcilytics). Journal of Molecular Endocrinology, 29, 15–21.
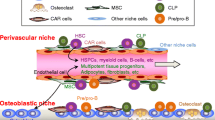
Strewler, G. J. (2006). The stem cell niche and bone metastasis. BoneKEy-Osteovision, 3, 19–29.
Sohara, Y., Shimada, H., Minkin, C., Erdreich-Epstein, A., Nolta, J. A., & DeClerck, Y. A. (2005). Bone marrow mesenchymal stem cells provide an alternate pathway of osteoclast activation and bone destruction by cancer cells. Cancer Research, 65, 1129–1135.
Neiva, K., Sun, Y. X., & Taichman, R. S. (2005). The role of osteoblasts in regulating hematopoietic stem cell activity and tumor metastasis. Brazilian Journal of Medical and Biological Research, 38, 1449–1454.
Calvi, L. M., Adams, G. B., Weibrecht, K. W., Weber, J. M., Olson, D. P., Knight, M. C., et al. (2003). Osteoblastic cells regulate the haematopoietic stem cell niche. Nature, 425, 841–846.
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., & Clarke, M. F. (2003). Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 100, 3983–3988.
Pantel, K., & Brakenhoff, R. H. (2004). Dissecting the metastatic cascade. Nature Reviews. Cancer, 4, 448–456.
Muller, V., & Pantel, K. (2004). Bone marrow micrometastases and circulating tumor cells: Current aspects and future perspectives. Breast Cancer Research, 6, 258–261.
Fournier, P. G., Chirgwin, J. M., & Guise, T. A. (2006). New insights into the role of T cells in the vicious cycle of bone metastases. Current Opinion in Rheumatology, 18, 396–404.
Weitzmann, M. N., & Pacifici, R. (2005). The role of T lymphocytes in bone metabolism. Immunological Reviews, 208, 154–168.
Stanley, K. T., VanDort, C., Motyl, C., Endres, J., & Fox, D. A. (2006). Immunocompetent properties of human osteoblasts: Interactions with T lymphocytes. Journal of Bone and Mineral Research, 21, 29–36.
Roato, I., Grano, M., Brunetti, G., Colucci, S., Mussa, A., Bertetto, O., et al. (2005). Mechanisms of spontaneous osteoclastogenesis in cancer with bone involvement. FASEB Journal, 19, 228–230.
Siegel, P. M., & Massague, J. (2003). Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nature Reviews. Cancer, 3, 807–821.
Bosma, G. C., Custer, R. P., & Bosma, M. J. (1983). A severe combined immunodeficiency mutation in the mouse. Nature, 301, 527–530.
Morrison, J., Partridge, T., & Bou-Gharios, G. (2005). Nude mutation influences limb skeletal muscle development. Matrix Biology, 23, 535–542.
Keuren, J. F., Magdeleyns, E. J., Govers-Riemslag, J. W., Lindhout, T., Curvers, J. (2006). Effects of storage-induced platelet microparticles on the initiation and propagation phase of blood coagulation. British Journal of Haematology, 134, 307–313.
Palumbo, J. S., Talmage, K. E., Massari, J. V., La Jeunesse, C. M., Flick, M. J., Kombrinck, K. W., et al. (2005). Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood, 105, 178–185.
Boucharaba, A., Serre, C. M., Gres, S., Saulnier-Blache, J. S., Bordet, J. C., Guglielmi, J., et al. (2004). Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. Journal of Clinical Investigation, 114, 1714–1725.
Boucharaba, A., Serre, C. M., Guglielmi, J., Bordet, J. C., Clezardin, P., & Peyruchaud, O. (2006). The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proceedings of the National Academy of Sciences of the United States of America, 103, 9643–9648.
Manabe, Y., Toda, S., Miyazaki, K., & Sugihara, H. (2003). Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer–stromal cell interactions. Journal of Pathology, 201, 221–228.
Calle, E. E., & Thun, M. J. (2004). Obesity and cancer. Oncogene, 23, 6365–6378.
Elliott, B. E., Tam, S. P., Dexter, D., & Chen, Z. Q. (1992). Capacity of adipose tissue to promote growth and metastasis of a murine mammary carcinoma: Effect of estrogen and progesterone. International Journal Cancer, 51, 416–424.
Iyengar, P., Combs, T. P., Shah, S. J., Gouon-Evans, V., Pollard, J. W, Albanese C., et al. (2003). Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene, 22, 6408–6423.
Somasundar, P., McFadden, D. W., Hileman, S. M., & Vona-Davis, L. (2004). Leptin is a growth factor in cancer. Journal of Surgical Research, 116, 337–349.
Maurin, A. C., Chavassieux, P. M., Frappart, L., Delmas, P. D., Serre, C. M., & Meunier, P. J. (2000). Influence of mature adipocytes on osteoblast proliferation in human primary cocultures. Bone, 26, 485–489.
Thomas, T., Gori, F., Khosla, S., Jensen, M. D., Burguera, B., & Riggs, B. L. (1999). Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology, 140, 1630–1638.
Parr, C., & Jiang, W. G., (2006). Hepatocyte growth factor activation inhibitors (HAI-1 and HAI-2) regulate HGF-induced invasion of human breast cancer cells. International Journal of Cancer, 119, 1176–1183.
Maeda, T., Alexander, C. M., & Friedl, A. (2004). Induction of syndecan-1 expression in stromal fibroblasts promotes proliferation of human breast cancer cells. Cancer Research, 64, 612–621.
Maeda, T., Desouky, J., & Friedl, A. (2006). Syndecan-1 expression by stromal fibroblasts promotes breast carcinoma growth in vivo and stimulates tumor angiogenesis. Oncogene, 25, 1408–1412.
Saad, S., Gottlieb, D. J., Bradstock, K. F., Overall, C. M., & Bendall, L. J. (2002). Cancer cell-associated fibronectin induces release of matrix metalloproteinase-2 from normal fibroblasts. Cancer Research, 62, 283–289.
Nguyen, N., Kuliopulos, A., Graham, R. A., & Covic, L. (2006). Tumor-derived Cyr61(CCN1) promotes stromal matrix metalloproteinase-1 production and protease-activated receptor 1-dependent migration of breast cancer cells. Cancer Research, 66, 2658–2665.
Delany, A. M., & Canalis, E. (2001). The metastasis-associated metalloproteinase stromelysin-3 is induced by transforming growth factor-beta in osteoblasts and fibroblasts. Endocrinology, 142, 1561–1566.
Lau, Y. S., Sabokbar, A., Giele, H., Cerundolo, V., Hofstetter, W., & Athanasou, N. A. (2006). Malignant melanoma and bone resorption. British Journal of Cancer, 94, 1496–1503.
Chavez-Macgregor, M., Aviles-Salas, A., Green, D., Fuentes-Alburo, A., Gomez-Ruiz, C., & Aguayo, A. (2005). Angiogenesis in the bone marrow of patients with breast cancer. Clinical Cancer Research, 11, 5396–5400.
Oehler, M. K., Hague, S., Rees, M. C., & Bicknell, R. (2002). Adrenomedullin promotes formation of xenografted endometrial tumors by stimulation of autocrine growth and angiogenesis. Oncogene, 21, 2815–2821.
Menendez, J. A., Mehmi, I., Griggs, D. W., & Lupu, R. (2003). The angiogenic factor CYR61 in breast cancer: Molecular pathology and therapeutic perspectives. Endocrine Related Cancer, 10, 141–152.
Wang, X. B., Yang, Q. X., & Pei, X. J. (2006). Expression of angiogenesis-related factors in invasive breast cancer and its clinical significance. Nan Fang Yi Ke Da Xue Xue Bao, 26, 860–863 (Article in Chinese).
Li, Y., Tondravi, M., Liu, J., Smith, E., Haudenschild, C. C., Kaczmarek, M., et al. (2001). Cortactin potentiates bone metastasis of breast cancer cells. Cancer Research, 61, 6906–6911.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siclari, V., Guise, T. & Chirgwin, J. Molecular interactions between breast cancer cells and the bone microenvironment drive skeletal metastases. Cancer Metastasis Rev 25, 621–633 (2006). https://doi.org/10.1007/s10555-006-9023-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-006-9023-1