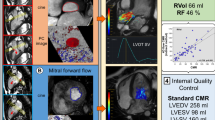
Abstract
Cardiac magnetic resonance (CMR) four-dimensional (4D) flow is a novel method for flow quantification potentially helpful in management of mitral valve regurgitation (MVR). In this systematic review, we aimed to depict the clinical role of intraventricular 4D-flow in MVR. The reproducibility, technical aspects, and comparison against conventional techniques were evaluated. Published studies on SCOPUS, MEDLINE, and EMBASE were included using search terms on 4D-flow CMR in MVR. Out of 420 screened articles, 18 studies fulfilled our inclusion criteria. All studies (n = 18, 100%) assessed MVR using 4D-flow intraventricular annular inflow (4D-flowAIM) method, which calculates the regurgitation by subtracting the aortic forward flow from the mitral forward flow. Thereof, 4D-flow jet quantification (4D-flowjet) was assessed in 5 (28%), standard 2D phase-contrast (2D-PC) flow imaging in 8 (44%) and the volumetric method (the deviation of left ventricle stroke volume and right ventricular stroke volume) in 2 (11%) studies. Inter-method correlations among the 4 MVR quantification methods were heterogeneous across studies, ranging from moderate to excellent correlations. Two studies compared 4D-flowAIM to echocardiography with moderate correlation. In 12 (63%) studies the reproducibility of 4D-flow techniques in quantifying MVR was studied. Thereof, 9 (75%) studies investigated the reproducibility of the 4D-flowAIM method and the majority (n = 7, 78%) reported good to excellent intra- and inter-reader reproducibility. Intraventricular 4D-flowAIM provides high reproducibility with heterogeneous correlations to conventional quantification methods. Due to the absence of a gold standard and unknown accuracies, future longitudinal outcome studies are needed to assess the clinical value of 4D-flow in the clinical setting of MVR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Mitral valve regurgitation (MVR) is one of the most common valvular heart diseases in western countries and its quantification is challenging due its complex geometry [1]. An accurate assessment of MVR however is crucial for patient risk stratification and optimal decision making towards mitral valve surgery. Furthermore, with the increasing availability of minimally invasive transcatheter treatment options, such as mitral valve transcatheter edge-to-edge repair (TEER), exact quantification of MVR severity and the identification of the underlying mechanism is key for identifying patients who can benefit from less invasive approaches and obviate the need for open heart surgery [2]. Moreover, MVR in hypertrophic cardiomyopathy (HCM) and primary valve disease such as mitral prolapse is still a clinical challenge. In clinical routine, transthoracic and transesophageal echocardiography (TOE) are the primary imaging modalities evaluating MVR and offer the possibility to determine a large number of qualitative (mitral valve leaflet and annular morphology, regurgitant jet size and location) and (semi-) quantitative parameters (vena contracta, regurgitate orifice, fraction and volume) of MVR severity [3]. Nevertheless, the comprehensive echocardiographic evaluation of MVR remains challenging due to the accurate and user dependent positioning of the echo probe, which is prone to bias [3, 4], and Cavalcante et al. [6] and Uretsky et al. [5] have shown in their studies that MVR assessed by cardiac magnetic resonance imaging (CMR) is more reliable than echocardiography in predicting patient outcomes after mitral valve repair.
Four-dimensional (4D) flow CMR is an emerging technology that combines the excellent soft-tissue delineation of conventional CMR with the velocity-encoded quantification of blood flow in three spatial directions [7]. Therefore, in comparison to two-dimensional phase-contrast (2D-PC) CMR, 4D-flow CMR is a potentially more consistent method for flow quantification. 4D-flow can assess blood flow not only across the large vessels but also through cardiac valves and ventricles. Several studies described an association of 4D-flow parameters to hemodynamic characteristics, implicating that 4D-flow is helpful in the evaluation of complex flow conditions such as left ventricular outflow track (LVOT)-obstruction in hypertrophic cardiomyopathy (HCM) [8], atrio-ventricular septal defect repair [9,10,11], or after valvular heart surgery [12]. Whether 4D-flow might also be used to accurately assess MVR has been evaluated in a few studies [13]. The aim of this systematic review was to identify the potential clinical role of intraventricular 4D-flow in MVR. Furthermore, the reproducibility, technical aspects and comparison against conventional techniques were assessed.
Methods
Two independent reviewers (i.e., authors YS and BB) conducted a systematic review on the database SCOPUS, MEDLINE and EMBASE database by reading the titles and abstracts [14]. To capture the full spectrum of 4D-flow CMR in MVR quantification, a search matrix with the following combinations of keywords was applied for English original articles, from 2010 until 2021: ((4D) OR (four-dimensional)) AND (flow) AND ((cardiac magnetic resonance imaging) OR (cardiovascular magnetic resonance imaging) OR (magnetic resonance imaging) OR (CMR) OR (MRI)) AND ((mitral valve) OR (left atrioventricular)) AND (regurgitation) OR (insufficiency). Inclusion criteria were the employment of 4D-flow CMR in the evaluation of MVR published in a full-text article until December 2021. The search was done at January 2022. This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement for reporting systematic reviews [15]. Due to the small number of studies and high heterogeneity in their methodology, a meta-analysis was not conducted.
Results
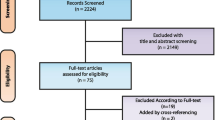
The initial search query yielded 420 articles. Based on the mentioned eligibility criteria, 29 articles remained potentially relevant to the current study (Fig. 1). After carefully reviewing the full manuscripts and excluding the studies using computational fluid dynamic (CFD) assessment (n = 3) or not assessing the MVR using 4D-flow methods (n = 8), a total of 18 studies were included in this systematic review, investigating the application of 4D-flow CMR in MVR. Most studies included (n = 12, 67%) were published after 2018, whereas 6 (33%) were studies published in or before 2017.
Consort flow of the study selection process. Flow diagram illustrating the stages of the systematic review process in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [15]. CMR, cardiovascular magnetic resonance imaging; CFD, computational fluid dynamics; MVR, mitral valve regurgitation
Study characteristics and aims
Baseline characteristics of the study cohorts, aim of the studies, publication year, and 4D-flow quantification methods are depicted in Table 1. The main objectives behind these studies were (1) to assess the accuracy and reproducibility of using 4D-flow CMR for quantifying MVR volume (n = 12, 67%), (2) to investigate the association of characteristics of the MVR jet with hemodynamic parameters (n = 3, 17%), and (3) to evaluate LV kinetic energy in patients with underlying cardiac disease and MVR (n = 3, 17%). Additionally, 11 studies (61%) compared patients with underlying cardiac disease and MVR to healthy volunteers for internal validity assessments. Across studies, underlying cardiac diseases such as mitral valve prolapse (MVP) [16], atrial fibrillation (AF) [17], and HCM [18] were included.
MVR quantification methods
MVR volume quantification methods require the assessment of stroke volume (SV) either by volumetrically using cine CMR images or by calculation from phase-contrast data. Figure 2 summarizes all the MVR volume quantification methods. (1) The “4D-intraventricular annular inflow method” (4D-flowAIM) calculates the regurgitant volume by subtracting the SV derived from aortic forward flow (SVAAo) from the SV derived from the forward flow through the mitral valve (SVMV), both derived from a single 4D-flow CMR dataset (available in n = 18 studies, 100%). The SVAAo and SVMV are calculated by integrating flows derived from the phase-contrast CMR images over the duration of one cardiac cycle. Additionally, (2) the clinical “2-dimensional phase-contrast standard method” (2D-PCstandard) is used to indirectly measure the MVR volume by subtracting the SV derived from PC imaging of SVAAo from volumetrically assed LV SV from cine CMR images (n = 8 studies, 44%). The LV SV is calculated by subtracting LV end-diastolic volume (EDV) from LV end-systolic volume (ESV) as derived from short axis cine images of the heart. The remaining methods are (3) “the volumetric method”, which calculates the deviation of the LV SV and right ventricular SV from cine CMR images in 2 (11%) studies, and (4) the 4D-flowjet method directly quantifying the flow and regurgitant volume of the regurgitant jet using 4D-flow CMR in 5 (28%) studies. No study assessed the MVR volume with (5) the “2D-PC mitral valve method” (2D-PCMV), which quantifies the MVR volume by subtracting SVMV from LV SV using 2D-PC and cine CMR images, analogous to the 2D-PCstandard method. It is important to note, that all quantification approaches, with the exception of the 2D-PCstandard method and 4D-flowjet method, require adaptation when significant aortic regurgitation is present. The replacement of the SV of the ascending aorta (AAo) or aortic valve (AoV) by the “net forward flow” through the AAo or AoV (calculated as the SV minus the volume of aortic regurgitation) allows proper quantification of MVR in these cases. Additionally, it is important to note that these methods have limited utility when there is interventricular shunting.
Illustration of MVR quantification methods. 2D-PCstandard, CMR flow gold standard (Left Ventricle Stroke Volume [LV SV]—Stroke Volume derived from Aortic Forward Flow [SVAAo]); 2D-PCMV, directly quantifying flow through Mitral Valve (Stroke Volume derived from Mitral Valve Flow [SVMV]—Stroke Volume derived from Aortic Forward Flow [SVAAo]); Volumetric (Left Ventricle Stroke Volume [LV SV]—Right Ventricle Stroke Volume [RV SV]); 4D-flowAIM (Stroke Volume derived from Mitral Valve Forward Flow [SVMV]—Stroke Volume derived from Aortic Forward Flow [SVAAo], or [SVLVOT]); 4D-flowjet; AoPC, Aortic Forward Flow; EDV, Left Ventricle End Diastolic Volume; ESV, Left Ventricle End Systolic
Technical parameters
Table 2 shows the technical parameters used in the reviewed studies. Scanners magnetic field strengths were 1.5 T (n = 11) and 3 T (n = 11). In all studies, the positioning of the FOV of the 4D-flow sequence was adapted to match a whole heart coverage, especially the entire left-sided cavities and the aortic root. The velocity encoding range (VENC) was set to values around 150 cm/s by default in most studies except in special cases such as congenital heart disease (CHD) [19]. The image resolution was ranging between 0.8 and 4.2 mm3, while most studies used a resolution of around 2.5 mm3, and a temporal resolution of around 40 ms (21–86 ms). Further acquisition parameters were as follows: echo time (TE) of 1–3 ms, repetition time (TR) of 5–15 ms, the flip angle was mostly 10° (7°–15°), and the mean image acquisition time was generally around 10 min (5–15 min). All studies administrated contrast agents before the 4D-flow acquisition, without specification of the exact timing, and used ECG triggering and respiratory gating. For flow analysis, retrospective valve tracking using post-processing software such as MASS (n = 6) (Leiden University Medical Center, The Netherlands) [9,10,11, 17, 20, 21] and cvi42 (n = 2) (Circle Cardiovascular Imaging, Calgary, Canada) [8, 18] was common. All studies visually assessed the quality of images and performed pre-processing for de-noising and anti-aliasing.
Reproducibility and comparison against other methods
Nine studies investigated the MVR quantification reproducibility of 4D-flowAIM, 7 studies (78%) reported good to excellent intra- and inter-reader reproducibility (ICC > 0.8) (Table 3), and the remaining 2 studies described good to excellent intra- but only moderate inter-reader reproducibility [8, 22]. None of the included studies have investigated the inter- and intra-scan reproducibility of 4D-flow acquisition. Seven studies (39%) investigated the agreement of 4D-flowAIM to other MVR acquisition methods [10, 16, 19, 20, 22,23,24] (Table 4). Inter-modality correlation among the 4 quantification methods was heterogeneous across studies, ranging from moderate to excellent correlation (r > 0.51). In direct comparison to 2D-PC, 4D-flowAIM measurements showed similar intra- and inter-observer agreement [16, 20]. Agreement of these techniques was also associated with the etiology of MVR. In primary MVR, a lower agreement (P > 0.05) was found compared to secondary MVR (P < 0.0001) [20]. When compared to the 2D-PC standard method, 4D-flowjet provided higher MVR volumes (P < 0.05) [20]. Two studies compared 4D-flowAIM to echocardiographic assessment of MVR volumes by the proximal isovelocity surface area (PISA) method with moderate correlation between the two modalities and systematically yielded higher MVR volumes as compared to CMR techniques (mean difference of 15.8 ml) [16].
Discussion
The findings of the current systematic review on 4D-flow for quantifying MVR volume are as follow: the reviewed studies demonstrated that 4D-flowAIM was the most common used quantification method in the setting of MVR and that the number of articles published are increasing in the recent five years. Moderate to strong agreement between different MVR quantification methods was depicted and reproducibility is generally high, and most authors concluded that 4D-flowAIM has the highest reproducibility across MVR quantification methods. So far, no study linked 4D-flow MVR quantifications to clinical outcomes.
Comparison of different MVR quantification methods
Due to its widespread availability, simplicity, and affordability, echocardiography by visual assessment and PISA method, remains the most popular modality to evaluate MVR severity. However, echocardiography has some constraints such as variable velocity assessment caused by beam alignment with non-optimal flow convergence, dynamic changes in orifice, limited acoustic window and operator experience. Further, in cases of multiple regurgitant orifices the PISA method is limited. Additionally, when complex flow patterns or complex vessel geometries are present, the calculation of mean velocities and net flow is frequently based on assumptions about the vessel's cross-sectional area or flow profile, which can lead to inaccurate flow quantifications, especially as the regurgitant orifice is not round, but rather oval or irregular in shape [7]. As a result, estimated echo velocity values have a moderate correlation with CMR quantitative measurements. Moreover, among CMR 4D-flow quantification methods might provide additional information with higher reproducibility and robustness in borderline moderate to severe MVR.
2D-PC CMR has become the reference gold standard for clinical aortic forward and backward flow (regurgitation) quantifications because of its high spatial and temporal resolution, simplicity in acquisition and post-processing, and good prognostic and diagnostic outcome data [27]. However, when used for MVR analysis, 2D-PC overestimates the MVR volume by 15% when compared to 4D-flowAIM [28] and is prone to errors because of the two different types of acquisition, 2D-PC and cine images [27]. Besides, concomitant valve disease might impact the accuracy of these measurements. Additionally, the 2D-PC imaging plane should be orthogonal to the flow direction, as stated by Vermes et al. in their study that the misalignment of the 2D-PC imaging plane prevents measuring the aortic peak velocity precisely and reduces the accuracy of flow measurements [29]. The CMR volumetric method based on one cine image acquisition allows a fast and easy assessment of MVR volumes and is a good method for quantifying solitary MVR. However, it is an indirect MVR quantification method, which has poor precision and high segmentation variability for right ventricle SV, and cannot be used in other valves incoherencies [27].
4D-flow CMR acquisitions allow for post-procedural adaptation of the angle and the position of the evaluation planes. 4D-flow has been used frequently for aortic diseases [30, 31], however, using the method in mitral valve disease is more complicated due to the saddle shape and significant through-plane motion of the mitral valve. To directly quantify the regurgitation jet volume with 4D-flowjet, proper cine image acquisitions and retrospective valve tracking (RVT) are required. Another advantage of 4D-flow quantification methods is their ability to enable direct valve tracking throughout the cardiac cycle, which is not feasible with 2D-flow imaging due to the motion of the valve annulus. This direct measurement capability is a significant advantage for assessing mitral regurgitation and allows for high reproducibility that might be superior to that of 2D PC methods [13, 23]. Nevertheless, the preferable MVR quantification method by CMR still has to be determined by systematic comparisons of reproducibility and robustness in intra- and inter-reader variability. Moreover, kinetic energy and wall shear stress are some advanced novel 4D-flow intraventricular hemodynamic parameters. For example, Gupta et al. [18] reported that left atrial kinetic energy assessed by 4D-flow is associated with LV obstruction in HCM patients. Whether these novel parameters maybe of advantage and may provide additional information in MVR with a potential clinical impact has to be evaluated in the future. Furthermore, there is no gold-standard MVR grading system by 4D-flow CMR, and the cut-off values are usually decided by the experts at each center. The consensus statement on assessing MVR by CMR suggested a grading system presented in Table 5 [27], however, further studies are required to compare the cut-off values for different quantification methods directly with outcomes.
Limitations of 4D-flow CMR in MVR
Across the reviewed studies, several limitations of 4D-flow CMR require attention, such as long acquisition time [11], using static time-averaged cine images for segmentations [8, 9, 11, 16, 18, 19, 26], difficulties in capturing the exact position of the peak MVR jet [10, 18, 19, 22], low temporal resolution in comparison to other CMR sequences, such as cine bSSFP [8, 20, 32], and the presence of image artifacts in patients with implanted devices [12].
Segmenting 4D-flow images based on time-averaged cine images requires an extra acquisition leading to misalignment between 4D-flow data and the cine images due to heart and patient movements [33]. Unfortunately, the blood-tissue contrast in 4D-flow is very low, which is why an accurate LV segmentation is difficult to perform on the 4D-flow data directly. Current approaches such as in Corrado et al. [34] register automated cine segmentations onto the 4D-flow data for faster analysis. Others, such as in Bustamante et al. [35] use atlas-based segmentations, that means a general segmentation mask is registered onto the 4D-flow CMR data and adapted to the scan. That atlas-based segmentation methods have been used to also train a U-net for direct LV segmentation of cardiac 4D-flow [36]. Prior research has shown that placing the atrioventricular plane at the position of the peak inflow velocity rather than at the height of the valvular plane improves the accuracy of 4D-flowAIM flow velocity estimation [9].
In Garcia et al. [37] a machine learning tool was developed to automatically detect evaluation planes following the mitral valve motion in cine data, which then were interpolated onto 4D-flow data. The need for a measuring plane perpendicular to valvular inflow likely extends to jet planes, which may explain the relatively poor correlation between mitral regurgitation fraction measurements using the volumetric, 4D-flowjet, and 4D-flowAIM techniques [19]. Moreover, the limited temporal resolution reduces the overall 4D-flow SNR [32] and affects the velocity profile quality [20] and the measured KE [38].
4D-flow acquisition parameters
4D-flow scanning parameters are dependent on many factors, such as the vendor, sequence, and patient’s hemodynamics, as indicated by the 4D-flow consensus statement [7]. The VENC (in cm/s) is often set to be 10% higher than the highest predicted velocity to achieve an acceptable velocity-to-noise ratio (VNR) and avoid aliasing. It is typically about 150 cm/s for MVR quantifications, ranging from 120 to 550 cm/s in the evaluated studies. Aliasing occurs when the VENC value is less than the highest flow velocity, and a high VENC results in a reduced VNR. The FOV of 4D-flow ideally covers the whole heart with the aortic arch. However, it is sufficient to cover the region of interest to decrease scan time, which in the case of MVR quantification is the left ventricle and left atrium. Since the spatial and temporal resolutions impact the accuracy of the flow acquisition, it is best to set them to the highest resolution if there is no time constraint. The temporal resolution is recommended to be lower than 40 ms as stated in the consensus [7], with a range of 21–86 ms. All the reviewed studies used retrospective ECG triggering to cover the whole cardiac cycle and avoid sequence interruptions. However, novel 4D-flow acquisitions use cardiac self-gating techniques [7]. All studies also used respiratory gating to decrease breathing artifacts and scan duration by positioning the navigator on the liver-diaphragm interface. Also, the flip angle varies from 5° to 15°. Overall, it can be concluded that variations in 4D-flow image quality might not be related to technique itself, rather to an inappropriate use of imaging parameters. A consensus of 4D-flow parameters for MVR is still needed.
As opposed to 2D-PC CMR, the 4D-flow analysis uses RVT to quantify eccentric regurgitation jets and correct for annular valve plane motions [10, 13, 26, 28]. In the net forward flow evaluation through cardiac valves, RVT has demonstrated greater accuracy with lesser variance when compared to 2D-PC CMR methods [10, 26, 28]. A multi-center study on assessing the consistency of automated RVT demonstrated that valvular flow measurement can be independent of local CMR scanners and protocols [25].
Even though the optimal setting for MVR quantification remains to be determined, currently used scanners and protocols, still allow for a consistent acquisition of 4D flow sequences [25].
Outlook on clinical implications
Data on the clinical value of MVR quantification by 4D-flow CMR is scarce and based on small observational studies. To the best of our knowledge, no study exists that links MVR characteristics determined by 4D-flow CMR to the long-term outcome or hard clinical endpoints such as mortality or heart failure events, or remodeling after mitral valve replacement. Conflicting data from large randomized clinical trials on the value of transcatheter mitral valve edge-to-edge repair [39, 40] underline the urgent need for a reproducible and robust quantification of MVR severity that correlates with outcomes and can be used to guide therapeutic decisions [41].
Limitations
When interpreting the results of this review, it is important to consider several limitations. The results presented show the current role of 4D-flow CMR in the assessment of MVR, which is currently based on descriptive, observational, and primarily retrospective data. The generalizability of our conclusions is reduced by the heterogeneity of the reviewed studies. Without considering factors such as the included study cohorts (healthy controls vs. patients with various cardiac diseases) [10, 12, 22, 32], the severity and mechanism of MVR, and various image acquisition techniques and analysis software packages, and the lack of a gold-standard, it is impossible to compare the values we provided for reproducibility and inter-modality correlation across studies. Further, how the use of contrast agent, the dosage and timing impacts on 4D flow quality is not yet conclusive and needs future evaluation. In addition to the mentioned limitations in the reviewed studies, it is noteworthy to consider the low availability of proper sequences and software in centers and a lack of clinical expertise restricting the broad adoption of clinical 4D-flow CMR [23].
Conclusions
Intraventricular 4D-flowAIM is the most used 4D-flow method in quantifying MVR among the reviewed studies providing high reproducibility with heterogeneous correlations to conventional quantification methods. Due to the absence of a gold standard, future longitudinal outcome studies need to assess the clinical value of different 4D-flow methods and compare its predictive value to established methods.
Availability of data and materials
The datasets analyzed during the current study are available via online search using Scopus and Google Scholar.
Abbreviations
- 2D-PC:
-
Two-dimensional phase-contrast
- AIM:
-
Annular inflow method
- CCT:
-
Cardiac computed tomography
- CMR:
-
Cardiac magnetic resonance imaging
- DICOM:
-
Digital imaging and communications in medicine
- HCM:
-
Hypertrophic cardiomyopathy
- KE:
-
Kinetic energy
- LVOT:
-
Left ventricular outflow track
- MVR:
-
Mitral valve regurgitation
- PISA:
-
Proximal isovelocity surface area
- RVT:
-
Retrospective valve tracking
- SV:
-
Stroke volume
- TEER:
-
Transcatheter edge-to-edge repair
- TOE:
-
Transesophageal echocardiography
- VENC:
-
Velocity encoding range
References
Iung B, Delgado V, Rosenhek R et al (2019) Contemporary presentation and management of valvular heart disease: the EURObservational research programme valvular heart disease II survey. Circulation 140:1156–1169
Otto CM, Nishimura RA, Bonow RO et al (2021) 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 77:e25–e197
Grayburn PA, Thomas JD (2021) Basic principles of the echocardiographic evaluation of mitral regurgitation. JACC Cardiovasc Imaging 14:843–853
Wang A, Grayburn P, Foster JA et al (2016) Practice gaps in the care of mitral valve regurgitation: Insights from the American College of Cardiology mitral regurgitation gap analysis and advisory panel. Am Heart J 172:70–79
Uretsky S, Animashaun IB, Sakul S et al (2022) American Society of Echocardiography algorithm for degenerative mitral regurgitation: comparison with CMR. Cardiovasc Imaging 15:747–760
Cavalcante JL, Kusunose K, Obuchowski NA et al (2020) Prognostic impact of ischemic mitral regurgitation severity and myocardial infarct quantification by cardiovascular magnetic resonance. Cardiovasc Imaging 13:1489–1501
Dyverfeldt P, Bissell M, Barker AJ et al (2015) 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson 17:72
Pruijssen JT, Allen BD, Barker AJ et al (2020) Hypertrophic cardiomyopathy is associated with altered left ventricular 3d blood flow dynamics. Radiol Cardiothor Imaging 2020:2
Calkoen EE, Roest AAW, Kroft LJM et al (2015) Characterization and improved quantification of left ventricular inflow using streamline visualization with 4DFlow MRI in healthy controls and patients after atrioventricular septal defect correction. J Magn Reson Imaging 41:1512–1520
Calkoen EE, Westenberg JJM, Kroft LJM et al (2015) Characterization and quantification of dynamic eccentric regurgitation of the left atrioventricular valve after atrioventricular septal defect correction with 4D Flow cardiovascular magnetic resonance and retrospective valve tracking. J Cardiovasc Magn Reson 2015:17
Calkoen EE, Elbaz MSM, Westenberg JJM et al (2015) Altered left ventricular vortex ring formation by 4-dimensional flow magnetic resonance imaging after repair of atrioventricular septal defects. J Thorac Cardiovasc Surg 150:1233-1240.e1
Morichi H, Itatani K, Yamazaki S et al (2020) Influences of mitral annuloplasty on left ventricular flow dynamics assessed with 3-dimensional cine phase-contrast flow magnetic resonance imaging. J Thorac Cardiovasc Surg 2020:589
Fidock B, Barker N, Balasubramanian N et al (2019) A systematic review of 4D-flow MRI derived mitral regurgitation quantification methods. Front Cardiovasc Med 6:103
Burnham JF (2006) Scopus database: a review. Biomed Digit Libr 3:1
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1-34
Spampinato RA, Jahnke C, Crelier G et al (2021) Quantification of regurgitation in mitral valve prolapse with four-dimensional flow cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2021:23
Mills MT, Grafton-Clarke C, Williams G et al (2021) Feasibility and validation of trans-valvular flow derived by four-dimensional flow cardiovascular magnetic resonance imaging in patients with atrial fibrillation. Wellcome Open Res 6:73–73
Gupta AN, Soulat G, Avery R et al (2021) 4D flow MRI left atrial kinetic energy in hypertrophic cardiomyopathy is associated with mitral regurgitation and left ventricular outflow tract obstruction. Int J Cardiovasc Imaging 2021:89
Jacobs K, Rigdon J, Chan F et al (2020) Direct measurement of atrioventricular valve regurgitant jets using 4D flow cardiovascular magnetic resonance is accurate and reliable for children with congenital heart disease: a retrospective cohort study. J Cardiovasc Magn Resonance 22:1
Fidock B, Archer G, Barker N et al (2021) Standard and emerging CMR methods for mitral regurgitation quantification. Int J Cardiol 331:316–321
Calkoen EE, De Koning PJH, Blom NA et al (2015) Disturbed intracardiac flow organization after atrioventricular septal defect correction as assessed with 4D flow magnetic resonance imaging and quantitative particle tracing. Invest Radiol 50:850–857
Blanken CPS, Westenberg JJM, Aben J-P et al (2020) Quantification of mitral valve regurgitation from 4D flow MRI using semiautomated flow tracking. Radiol Cardiothorac Imaging 2:e200004
Feneis JF, Kyubwa E, Atianzar K et al (2018) 4D flow MRI quantification of mitral and tricuspid regurgitation: reproducibility and consistency relative to conventional MRI. J Magn Reson Imaging 48:1147–1158
Hsiao A, Tariq U, Alley MT, Lustig M, Vasanawala SS (2015) Inlet and outlet valve flow and regurgitant volume may be directly and reliably quantified with accelerated, volumetric phase-contrast MRI. J Magn Reson Imaging 41:376–385
Juffermans JF, Minderhoud SCS, Wittgren J et al (2021) Multicenter consistency assessment of valvular flow quantification with automated valve tracking in 4D flow CMR. JACC Cardiovasc Imaging 14:1354
Kamphuis VP, Roest AAW, Marsan NA et al (2019) Automated cardiac valve tracking for flow quantification with four-dimensional flow MRI. Radiology 290:70–78
Garg P, Swift AJ, Zhong L et al (2020) Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nature Rev Cardiol Nature Res 2020:298–312
Westenberg JJ, Roes SD, Ajmone Marsan N et al (2008) Mitral valve and tricuspid valve blood flow: accurate quantification with 3D velocity-encoded MR imaging with retrospective valve tracking. Radiology 249:792–800
Vermes E, Iacuzio L, Levy F et al (2022) Role of cardiovascular magnetic resonance in native valvular regurgitation: a review of protocols, grading of severity and prediction of valve intervention. Front Cardiovasc Med 9:1695
Soulat G, Scott MB, Allen BD et al (2022) Association of regional wall shear stress and progressive ascending aorta dilation in bicuspid aortic valve. JACC Cardiovasc Imaging 15:33–42
Chu S, Kilinc O, Pradella M et al (2022) Baseline 4D flow-derived in vivo hemodynamic parameters stratify descending aortic dissection patients with enlarging aortas. Front Cardiovasc Med 9:905718
Arvidsson PM, Toger J, Pedrizzetti G et al (2018) Hemodynamic forces using four-dimensional flow MRI: an independent biomarker of cardiac function in heart failure with left ventricular dyssynchrony? Am J Physiol Heart Circ Physiol 315:H1627–H1639
Ma LE, Yerly J, Piccini D et al (2020) 5D flow MRI: a fully self-gated, free-running framework for cardiac and respiratory motion-resolved 3D hemodynamics. Radiol Cardiothorac Imaging 2:e200219
Corrado PA, Wentland AL, Starekova J, Dhyani A, Goss KN, Wieben O (2022) Fully automated intracardiac 4D flow MRI post-processing using deep learning for biventricular segmentation. Eur Radiol 32:1–10
Bustamante M, Gupta V, Forsberg D, Carlhäll C-J, Engvall J, Ebbers T (2018) Automated multi-atlas segmentation of cardiac 4D flow MRI. Med Image Anal 49:128–140
Bustamante M, Viola F, Engvall J, Carlhäll CJ, Ebbers T (2022) Automatic time-resolved cardiovascular segmentation of 4D flow MRI using deep learning. J Magn Reson Imaging 57:1
Garcia J, Beckie K, Hassanabad AF, Sojoudi A, White JA (2021) Aortic and mitral flow quantification using dynamic valve tracking and machine learning: prospective study assessing static and dynamic plane repeatability, variability and agreement. JRSM Cardiovasc Dis 10:2048004021999900
Steding-Ehrenborg K, Arvidsson PM, Toger J et al (2016) Determinants of kinetic energy of blood flow in the four-chambered heart in athletes and sedentary controls. Am J Physiol Heart Circ Physiol 310:H113–H122
Stone GW, Lindenfeld J, Abraham WT et al (2018) Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 379:2307–2318
Obadia JF, Messika-Zeitoun D, Leurent G et al (2018) Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 379:2297–2306
Al-Wakeel N, Fernandes JF, Amiri A, Siniawski H, Goubergrits L, Berger F, Kuehne T (2015) Hemodynamic and energetic aspects of the left ventricle in patients with mitral regurgitation before and after mitral valve surgery. J Magn Reson Imaging 42(6):1705–1712. https://doi.org/10.1002/jmri.24926
Acknowledgements
We would like to thank Lukas Lüthi for his administrative coordination and support in this study.
Funding
Open access funding provided by University of Bern. This work has been funded by the Swiss National Science foundation 197754.
Author information
Authors and Affiliations
Contributions
YS and BB were responsible for review of relevant literature, drafting the manuscript and preparation of tables and figures. BJ, EP, RK, and JB were responsible for revision of the draft manuscript. CG was responsible for revision and final approval of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
CG received research funding from the Swiss National Science Foundation [Nr 197754 and 200871]. Further, CG has received research funding from Innosuisse, Center of Artificial Intelligence in Medicine, University of Bern, Switzerland, and GAMBIT foundation. YS has received research funding from the Center of Artificial Intelligence in Medicine, University of Bern, Switzerland. BB received a career-development grant from the Swiss National Science Foundation. JB received funding from the Swiss National Science Foundation [Nr 194296]. All other authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Safarkhanlo, Y., Jung, B., Bernhard, B. et al. Mitral valve regurgitation assessed by intraventricular CMR 4D-flow: a systematic review on the technological aspects and potential clinical applications. Int J Cardiovasc Imaging 39, 1963–1977 (2023). https://doi.org/10.1007/s10554-023-02893-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-023-02893-z