Abstract
Assessment of left ventricular filling pressure (LVFP) is crucial in patients with ST-segment elevation myocardial infarction (STEMI). Since current guideline recommended echocardiographic parameters have limited value, more comprehensive assessment methods are required in this patient subset.In this study, we aimed to investigate the clinical utility of left atrial reservoir strain (LARS) imaging in patients treated with primary percutaneous coronary intervention (pPCI). Patients who underwent successful pPCI were included. Left ventricular end-diastolic pressure (LVEDP) was measured invasively following pPCI. Left atrial strain imaging was performed following pPCI within 24 h of pPCI. Normal LARS value was accepted as above 23%. We prospectively enrolled 69 patients; there were 18 patients with LARS below 23% who were included into group 1 and rest of the study population included into group 2. There was no significant difference between groups in terms of comorbidities.Troponin and pro-BNP levels were significantly higher in group 1 (p: 0.036 and 0.047 respectively). Left atrial volume and tricuspid regurgitation velocity were similar between groups (p: 0.416 and p: 0.351 respectively). Septal tissue velocity was higher (p: 0.001) and Septal E/e’ ratio was lower (p: 0.004) in group 2. Left ventricular (LV) global longitudinal strain value was higher in group 1 which is consistent with observed lower ejection (LVEF) fraction in group 1 (p: 0.001 for LV strain and p: 0.001 for LVEF). Estimated mean LVFP was also higher in group 1 (p: 0.003).Correlation analyses revealed moderate correlation between LARS and LVEDP (r: − 0.300). Our results indicate that left atrial strain imaging is a promising tool for the assessment of left atrial pressure in patients with STEMI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increased left ventricular filling pressure (LVFP) is one of the determinants of cardiovascular morbidity and mortality in patients with acute coronary syndrome (ACS), which was first emphasized by Killip et al. and subsequently by Forrester et al. who used pulmonary capillary wedge pressure as a surrogate of left atrial pressure [1, 2]. The inherent complications associated with its invasive nature makes routine assessment of pulmonary capillary wedge pressure (PCWP) by cardiac catheterization not feasible. Hence, in daily practice, non-invasive assessment of LVFP is the preferred method for its clinical evaluation. EUROFILLING study has validated the current guideline recommendations for measurement of LVFP, however patients with ACS were excluded [3, 4]. Therefore, echocardiographic parameters of increased LVFP in patients with ACS need to be clarified. Our group previously tested the aforementioned guideline recommendations and concluded that current echocardiographic parameters have limited value for the detection of increased LVFP in patients with acute coronary syndrome [5].
In patients with stable coronary artery disease, increased LVFP results in increased left atrial (LA) pressure. Left atrial remodelling in response to increased LA pressure occurs, and LA volume increases in the chronic phase. However, in patients with ACS, LVFP and subsequently LA pressure increases immediately following ischemia-induced myocardial dysfunction without required time for the LA enlargement. Therefore, in patients with ACS, LA volume and volume index are not a feasible measure of increased LVFP. Left atrial strain measurement is a relatively novel method for the detection of increased LVFP. Singh et al. demonstrated that peak LA strain is reduced significantly in patients with left ventricular diastolic dysfunction (LVDD) and concluded that as the severity of LVDD increases, peak LA strain is reduced [6]. Furthermore, Morris et al. investigated the additive value of LA strain on LA volume index (LAVI) for the detection of increased LVFP and concluded that abnormal LA strain is associated with increased estimated PCWP even in patients with normal LAVI [7].
In this study, our primary aim was to investigate the usefulness of LA strain in comparison with invasive assessment, for the detection of increased LVFP in patients with ST-segment elevation acute myocardial infarction (STEMI) who were treated with primary percutaneous intervention.
Materials and methods
Study protocol
We prospectively included patients with acute STEMI who were treated with primary percutaneous intervention in Cerrahpasa School of Medicine Hospital between July 2020 and October 2020. The local ethics committee of our institution approved the study protocol (21.07.2020, number: 83045809-604.01.02-). Written informed consent was obtained from each patient in accordance with declaration of Helsinki prior to inclusion. The inclusion criteria were as follows; (i) patients with a clear diagnosis of STEMI according to current guidelines [8], (ii) patients treated with successful primary PCI, (iii) patients older than 18 years. Patients were excluded from the study if one of the following was present; (i) previous diagnosis of heart failure, coronary artery disease or chronic renal disease ii) severe valvular heart disease, (iii) Killip III–IV presentation, (iv) refusal to give informed consent, (v) atrial fibrillation and sustained ventricular tachycardia/fibrillation and (vi) missing clinical data. Patients’ demographic and clinical history including comorbidities and features associated with myocardial infarction were recorded. The medical therapy in addition to antithrombotic medications used in the catheterization laboratory, consisted of angiotensin converting enzyme inhibitors or angiotensin receptor blockers, beta blocker and high dose statin which were initiated right after transfer to coronary care unit depending on patients’ haemodynamic status. Blood samples were obtained after the primary PCI for biochemical analyses for which a fasting status was not required.
Angiograpy procedure
Primary PCIs were performed using Philips Allura Exper (Philips, Amsterdam, The Netherlands) angiography system. All coronary angiographies and subsequent percutaneous coronary interventions and LV pressure measurements were performed by an experienced invasive cardiologist team (performing > 200 primary PCIs annually). The vast majority of patients were treated with stent implantation whereas patients with unsuitable anatomy for stenting were treated with balloon angioplasty in order to ensure distal flow and underwent to coronary by-pass grafting surgery for the intent of complete revascularization within first week of acute event. Left ventricular pressure recording was performed following primary PCI by advancing the right Judkins catheter into the LV, simultaneously with ECG recording. Normal threshold for left ventricular end-diastolic pressure was taken as < 18 mmHg which is the accepted normal range in Forrester classification. Pressure transducer was zeroed at mid-chest level prior to measurement in each patient. Left ventricular pressure at the time of electrocardiographic P wave onset was accepted as pre-A wave pressure and left ventricular pressure at the time of electrocardiographic R wave peak was accepted as left ventricular end-diastolic pressure.
Echocardiographic procedure
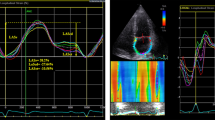
Patients underwent to echocardiographic evaluation within 24 h after primary PCI. Echocardiographic examinations were performed using a Philips EPIQ echocardiography machine (Philips Medical Systems, Andover, MA, USA) by two European Association of Cardiovascular Imaging board certified cardiologists. Left atrial volume was calculated using biplane Simpson’s method in apical 4-chamber view and 2-chamber view and indexed to body surface area. Left ventricular ejection fraction was also calculated using modified Simpson’s method. Septal e′ velocity was measured using tissue Doppler imaging. Estimated mean left atrial pressure was calculated using Nagueh formula described elsewhere [9]. Tricuspid regurgitation velocity was measured in multiple echocardiographic windows and the highest obtained velocity was used to calculate pulmonary artery systolic pressure. Left ventricular global longitudinal strain and left atrial strain measurements were performed as recommended by current consensus documents [10]. Strain analyses were made using acquired ECG gated images on the dedicated automatic border detecting program. Manual corrections of region of interest were made if deemed necessary. Normal left atrial reservoir strain (LARS) was accepted to be > 23% [11].
Statistical analyses
All statistical tests were conducted using the Statistical Package for the Social Sciences 21.0 for Windows (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov or Shapiro–Wilk tests was used to analyse normality of the data. Continuous data are expressed as mean ± SD and analysed using Mann–Whitney U tests. Categorical data are expressed as percentages and Chi-square or Fisher exact tests were used to assess differences in categorical variables between groups. The correlation between left atrial strain values and clinical parameters were tested using Spearman correlation analyses. Significance was assumed at a 2-sided p < 0.05. 15 patients were randomly selected to test the intra- and inter-observer reproducibility of LA strain and LV filling pressure measurements and tested using Bland–Altman analysis.
Results
We prospectively screened 133 patients with STEMI. Sixty four patients were excluded from the study: 4 patients died during index hospitalization, 8 patients had previous diagnosis of heart failure, 31 patients had previous diagnosis of coronary artery disease, 9 patients were found to be Killip III/IV on presentation and 4 patients had persistent atrial fibrillation. A further batch of 8 patients were excluded due to poor image quality and final analyses were carried out in the remaining 69 patients. There were 18 patients with LARS below 23%, which constituted group 1, and rest of the patients were included in the group 2.
Patient demographics
Table 1 demonstrates the comparison of clinical characteristics. Study population was relatively young and there was no significant difference between groups (p: 0.102). The prevalence of comorbidities including hypertension, diabetes mellitus and dyslipidaemia were also similar between the groups (p = 0.898 for hypertension, p = 0.358 for diabetes, p = 0.067 for dyslipidaemia). Despite numerically higher percentage of anterior myocardial infarction in group 1, no statistically significant difference was observed between groups in terms of the incidence of anterior myocardial infarction (p = 0.399), as it was for multivessel coronary artery disease (p = 0.761). Peak troponin levels and pro-BNP levels were higher in group 1 patients (p = 0.036 for troponin and p = 0.047 for pro-BNP). Although mean total ischemic time was 90 min longer in group 1 this difference did not reach to statistical significance (p = 0.627). Left ventricular end-diastolic pressure was below 18 among 41 patients and significantly higher in group 1 patients compared to group 2 patients [19.15 (12.4–32.8) mmHg vs 16.7 (9–25.9) mmHg, p: 0.003] (Fig. 1).
Echocardiography
Comparison of echocardiographic parameters was depicted in Table 2. Left ventricular ejection fraction was significantly lower in group 1 patients (p = 0.001). Consistent with LVEF, left ventricular global strain was higher in this patient group (p = 0.001). LAVI was within the normal range in majority of the study population and there was no significant difference between groups (p = 0.428). In addition, tricuspid regurgitation velocity (TRV) and mitral flow E wave velocity were also comparable between groups (p = 0.77 for mitral E velocity and p = 0.351 for TRV). However, septal e′ velocity was statistically lower in group 1 patients (0 = 001). Concordant with septal e′ velocity, septal E/e′ ratio was significantly higher in group 1 patients (p = 0.004). Mean left atrial pressure which calculated using Nagueh formula was higher in group 1 patients (p = 0.003). Receiver operating characteristics (ROC) analysis demonstrated that LARS value above 34% significantly predicts normal LVEDP (area under the curve: 0.688, sensitivity: 51.2%, specificity: 76.9 and p: 0.018) (Fig. 2).
Correlation analyses
Correlation analyses demonstrated that left atrial strain value is moderately correlated with left ventricular end-diastolic pressure (Table 3). Moreover, there were also significant correlations between left atrial strain and left ventricular systolic parameters in terms of LVEF and left ventricular GLS (r: 0.415 for LVEF and r: 0.314 for GLS). Among parameters of increased left atrial pressure, tricuspid regurgitation velocity, septal e′ velocity and E/e′ ratio were correlated with left atrial strain (r = 0.263 for TRV, r: 0.265 for septal e′ and r = 0.245 for septal E/e′ ratio). However, there was no correlation between left atrial volume index and left atrial strain values.
Reproducibility
Randomly selected 15 patients were investigated in order to assess reproducibility of LA strain imaging and LV filling pressure analyses. There were excellent inter-observer correlations between observers for both LA strain imaging analyses and LV filling pressure analyses (r: 0.93 and r: 0.95 respectively).
Discussion
To the best of our knowledge, this is the first study investigating the value of left atrial strain imaging for the assessment of LVFP in comparison to invasively measured left ventricular end-diastolic pressure in patients with STEMI. Our principal findings are; (i) LARS is moderately correlated with LVEDP, (ii) LARS above 34% may be useful for the exclusion of increased LVEDP and (iii) LARS is also associated with echocardiographic left ventricular systolic function indicators.
Acute ST-segment elevation myocardial infarction has a sudden impact on the left ventricle and results in both systolic and diastolic dysfunction, both adversely affecting clinical outcomes [12]. Compared to systolic function, which is relatively easy to assess, diastolic dysfunction during the recovery phase of STEMI is arbitrary. Since the guideline-recommended echocardiographic parameters indicating increased LV filling pressure depend on presence of the compensatory response of heart chambers to chronic pressure overload, acute haemodynamic impact of STEMI on left ventricle and subsequent reflection on the left atrium complicates the non-invasive assessment of LVFPs. We have previously tested the parameters of increased LVFP according to current guidelines and demonstrated the limitations of aforementioned criteria in patients with STEMI. In this study, we have tested whether left atrial strain imaging is feasible in detecting increased LVFP in patients with STEMI who were treated with primary PCI. Our results indicated that decreased LARS indicates increased LVFP, which was quantitatively assessed with invasive measurement of LVEDP following primary PCI. Previously, Li-Fan et al. demonstrated that LARS has good correlation (r = 0.66) with invasively measured LVEDP in patients with preserved LV systolic function [13]. We have demonstrated a similar albeit weaker correlation between these two parameters in our study. A possible explanation for this discrepancy between Li-Fan et al. findings and ours may be the difference between study populations in terms of LV systolic functions. EUROFILLING study, which validated the current echocardiographic algorithm, demonstrated that although diastolic dysfunction algorithm correlates well with invasively measured LV pressure, correlation was stronger in patients with preserved LV systolic function [3]. Singh et al. suggested that LARS as a single measure could be valuable to assess LV diastolic functions which is validated with LV pressure measurement, however excluded patients with acute STEMI [14]. Our results suggest that LARS may also be a feasible tool to detect increased LVFP in patients with STEMI.
The findings of the present study also indicate that left atrial strain imaging is associated with the parameters of left ventricular systolic function, which were assessed with LVEF and GLS. Considering the fact, that LA reservoir phase depends on LA relaxation and downward movement of mitral annulus during LV contraction, our findings are logical. Barbier et al. studied ischemia-induced pig models and demonstrated the association between LA reservoir functions and LV systolic functions [15]. Ersboll et al. also demonstrated that left atrial peak strain is strongly correlated with left ventricular global strain in patients with STEMI which is also in line with our results [16]. Moreover, peak troponin and pro-BNP levels, which are indicators of extent of myocardial injury, are higher in patients with reduced LARS. This is also concordant with the observed association between LARS and LV systolic functions. There was no significant difference between groups with respect to left atrial volume index and LAVI was not correlated with LARS in our study. Since left atrial volume expansion is a response to chronic pressure overload, it is not expected to be observed in patients with acute STEMI despite the increased LV filling pressures. Septal e′ velocity reflects both systolic and diastolic functions and we demonstrated significantly decreased septal e′ velocity in patients with reduced LARS. Although correlation was modest between septal e′ and LARS, it is concordant with decreased LV systolic functions and increased LVEDP in this patient group.
In conclusion, our results indicate that LARS is a feasible and reproducible non-invasive method for the identification of patients with increased LVP in the setting of acute STEMI. As demonstrated earlier, since LA reservoir functions are affected by both LA relaxation properties and LV systolic functions, our results are in line with previous reports. Further studies with larger study population and longer follow-up duration are necessary in order to demonstrate the clinical value of LARS in terms of prognosis in STEMI patients.
Limitations
Major limitation of our study is the absence of outcome data. Although we analysed in- hospital outcomes of patients and observed a significantly higher prevalence of heart failure, due to the limited number of patients with heart failure [6 (33.3%) patients in group 1 vs 9 (17.6%) patients in group 2] we have opted not to include the numbers in the result section. In addition, 4 new-onset atrial fibrillation was observed during the study, all of which recorded in group 1 patients. In addition exclusion of patients with Killip III and IV presentation should have underrepresentation of patients with increased LVEDP. Our study has relatively small number of patients, however due to strict exclusion criteria and unprecedented COVID-19 pandemic, patient enrolment was slower than our expectations. Last, instead of a curved catheter such as Pigtail catheter, we measured the LV pressures using Judgkins Right catheter which requires significant attention in order to avoid measurement errors.”
References
Killip T, Kimball JT (1967) Treatment of myocardial infarction in a coronary care unit. A Two year experience with 250 patients. Am J Cardiol 20:457–464. https://doi.org/10.1016/0002-9149(67)90023-9
Forrester J, Diamond G, Swan HJC (1977) Correlative classification of clinical and hemodynamic function after acute myocardial infarction. Am J Cardiol 39:137–145
Lancellotti P, Galderisi M, Edvardsen T et al (2017) Echo-Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro-Filling study. Eur Heart J Cardiovasc Imaging 18:961–968. https://doi.org/10.1093/ehjci/jex067
Nagueh SF, Smiseth OA, Appleton CP et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29:277–314. https://doi.org/10.1016/j.echo.2016.01.011
Durmaz E, Ikitimur B, Karadag B et al (2021) Echocardiographic assessment of left ventricular filling pressure in patients with acute ST elevation myocardial infarction: an invasive validation study. Int J Cardiovasc Imaging 37:1587–1594. https://doi.org/10.1007/S10554-020-02138-3
Singh A, Addetia K, Maffessanti F et al (2017) LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging 10:735–743. https://doi.org/10.1016/J.JCMG.2016.08.014
Morris DA, Belyavskiy E, Aravind-Kumar R et al (2018) Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging 11:1405–1415. https://doi.org/10.1016/J.JCMG.2017.07.029
Arslan F, Bongartz L, ten Berg JM et al (2018) 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: comments from the Dutch ACS working group. Netherlands Heart J 26:417–421. https://doi.org/10.1007/s12471-018-1134-0
Nagueh S, Middleton K et al (1997) Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am College Cardiol 30(6):1527–1533
Badano LP, Kolias TJ, Muraru D et al (2018) Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Hear J Cardiovasc Imaging 19:591–600. https://doi.org/10.1093/EHJCI/JEY042
Morris D, Takeuchi M et al (2015) Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging 16(4):364–372
Prasad SB, See V, Brown P et al (2011) Impact of duration of ischemia on left ventricular diastolic properties following reperfusion for acute myocardial infarction. Am J Cardiol 108:348–354. https://doi.org/10.1016/J.AMJCARD.2011.03.051
Fan J-L, Su B, Zhao X et al (2020) Correlation of left atrial strain with left ventricular end-diastolic pressure in patients with normal left ventricular ejection fraction. Int J Cardiovasc Imaging 36:1659–1666. https://doi.org/10.1007/s10554-020-01869-7
Singh A, Medvedofsky D, Mediratta A et al (2019) Peak left atrial strain as a single measure for the non-invasive assessment of left ventricular filling pressures HHS Public Access. Int J Cardiovasc Imaging 35:23–32. https://doi.org/10.1007/s10554-018-1425-y
Barbier P, Solomon SB, Schiller NB, Glantz SA (1999) Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 100:427–436. https://doi.org/10.1161/01.CIR.100.4.427
Ersbøll M, Andersen MJ, Valeur N et al (2013) The prognostic value of left atrial peak reservoir strain in acute myocardial infarction is dependent on left ventricular longitudinal function and left atrial size. Circ Cardiovasc Imaging 6:26–33. https://doi.org/10.1161/CIRCIMAGING.112.978296
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Durmaz, E., Karpuz, M.H., İkitimur, B. et al. The validation of left atrial strain imaging for the assessment of diastolic functions in patients with ST-segment elevation myocardial infarction. Int J Cardiovasc Imaging 38, 2109–2114 (2022). https://doi.org/10.1007/s10554-022-02628-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-022-02628-6