Abstract
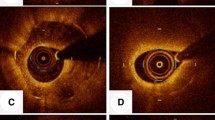
The purpose of this study was to evaluated the clinical characteristics of calcified nodule-like in-stent restenosis (ISR) lesions using optical coherence tomography (OCT) in vivo. A total of 124 ISR lesions that were treated with a repeat coronary intervention under OCT guidance were included in this analysis. ISR neointimal morphology was classified as “calcified nodule-like ISR”, that appeared as a high-backscattering protruding mass with an irregular surface covered by signal-rich bands, or “non-calcified nodule-like ISR”. The maximum arc and thickness of calcium behind the stent struts was also measured. Of the 124 ISR lesions, calcified nodule-like ISR was observed in 11 lesions (9%). OCT analysis data showed that the maximum arc of calcium and the maximum calcium thickness behind the stent were significantly larger in the calcified nodule-like ISR lesions than in the non-calcified nodule-like ISR lesions (269 ± 51 vs. 179 ± 92°, p < 0.01 and 989 ± 174 vs. 684 ± 241 μm, p < 0.01, respectively). The enlargement of the stent area was significantly larger in the calcified nodule-like ISR lesions than in the non-calcified nodule-like ISR lesions (1.6 ± 2.3 vs. 0.7 ± 1.3 mm2, p = 0.02). As a result, the enlargement of the lumen area tended to be larger in the calcified group (2.8 ± 1.7 vs. 2.4 ± 1.3 mm2, p = 0.3). Calcified nodule-like neointima within the stent could develop in approximately 10% of all ISR lesions, especially within stents deployed in severely calcified lesions.


Similar content being viewed by others
References
Vavuranakis M, Toutouzas K, Stefanadis C, Chrisohou C, Markou D, Toutouzas P (2001) Stent deployment in calcified lesions: can we overcome calcific restraint with high-pressure balloon inflations? Catheter Cardiovasc Interv 52:164–172
Mosseri M, Satler LF, Pichard AD, Waksman R (2005) Impact of vessel calcification on outcomes after coronary stenting. Cardiovasc Revasc Med 6:147–153
Doi H, Maehara A, Mintz GS, Yu A, Wang H, Mandinov L et al (2009) Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: an integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc Interv 2:1269–1275
Liu X, Doi H, Maehara A, Mintz GS, Costa JR, Sano K et al (2009) A volumetric intravascular ultrasound comparison of early drug-eluting stent thrombosis versus restenosis. JACC Cardiovasc Interv 2:428–434
Kawaguchi R, Tsurugaya H, Hoshizaki H, Toyama T, Oshima S, Taniguchi K (2008) Impact of lesion calcification on clinical and angiographic outcome after sirolimus-eluting stent implantation in real-world patients. Cardiovasc Revasc Med 9:2–8
Bangalore S, Vlachos HA, Selzer F, Wilensky RL, Kip KE, Williams DO et al (2011) Percutaneous coronary intervention of moderate to severe calcified coronary lesions: insights from the National Heart, Lung, and Blood Institute Dynamic Registry. Catheter Cardiovasc Interv 77:22–28
Bastante T, Rivero F, Cuesta J, Alfonso F (2015) Calcified neoatherosclerosis causing "undilatable" in-stent restenosis: insights of optical coherence tomography and role of rotational atherectomy. JACC Cardiovasc Interv 8:2039–2040
Koga S, Ikeda S, Nakata T, Kawano H, Abe K, Maemura K (2015) Diverse findings in calcified thrombus between histopathology and in vivo imaging including intravascular ultrasound, optical coherence tomography, and angioscopy. Int Heart J 56:661–663
Alfonso F, Cuesta J, Bastante T, Aguilera MC, Benedicto A, Rivero F (2016) In-stent restenosis caused by a calcified nodule: a novel pattern of neoatherosclerosis. Can J Cardiol 32:830.e1–3
Mori H, Finn AV, Atkinson JB, Lutter C, Narula J, Virmani R (2016) Calcified nodule: an early and late cause of in-stent failure. JACC Cardiovasc Interv 9:e125–126
Ellis SG, Vandormael MG, Cowley MJ, DiSciascio G, Deligonul U, Topol EJ et al (1990) Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation 82:1193–1202
Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF et al (1999) Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation 100:1872–1878
Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC et al (1995) Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation 91:1959–1965
Lee T, Mintz GS, Matsumura M, Zhang W, Cao Y, Usui E et al (2017) Prevalence, predictors, and clinical presentation of a calcified nodule as assessed by optical coherence tomography. JACC Cardiovasc Imaging 10:883–891
Fujii K, Masutani M, Okumura T, Kawasaki D, Akagami T, Ezumi A et al (2008) Frequency and predictor of coronary thin-cap fibroatheroma in patients with acute myocardial infarction and stable angina pectoris a 3-vessel optical coherence tomography study. J Am Coll Cardiol 52:787–788
Takarada S, Imanishi T, Liu Y, Ikejima H, Tsujioka H, Kuroi A et al (2010) Advantage of next-generation frequency-domain optical coherence tomography compared with conventional time-domain system in the assessment of coronary lesion. Catheter Cardiovasc Interv 75:202–206
Gonzalo N, Serruys PW, Okamura T, van Beusekom HM, Garcia-Garcia HM, van Soest G et al (2009) Optical coherence tomography patterns of stent restenosis. Am Heart J 158:284–293
Fujii K, Kubo T, Otake H, Nakazawa G, Sonoda S, Hibi K et al (2019) Expert consensus statement for quantitative measurement and morphological assessment of optical coherence tomography. Cardiovasc Interv Ther 35:13–18
Kanda Y (2013) Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48:452–458
Otsuka F, Joner M, Prati F, Virmani R, Narula J (2014) Clinical classification of plaque morphology in coronary disease. Nat Rev Cardiol 11:379–389
Saita T, Fujii K, Hao H, Imanaka T, Shibuya M, Fukunaga M et al (2017) Histopathological validation of optical frequency domain imaging to quantify various types of coronary calcifications. Eur Heart J Cardiovasc Imaging 18:342–349
Nakano M, Yahagi K, Yamamoto H, Taniwaki M, Otsuka F, Ladich ER et al (2016) Additive value of integrated backscatter IVUS for detection of vulnerable plaque by optical frequency domain imaging: an ex vivo autopsy study of human coronary arteries. JACC Cardiovasc Imaging 9:163–172
Kawai K, Akahori H, Imanaka T, Miki K, Yoshihara N, Yanaka K et al (2019) Coronary restenosis of in-stent protruding bump with rapid progression: optical frequency domain imaging and angioscopic observation. J Cardiol Cases 19:12–14
Kato Y, Iwata A, Nakamura M, Miura SI, Saku K (2017) In-stent restenosis due to stent recoil after third-generation drug-eluting stent implantation. J Clin Med Res 9:534–538
Yamamoto W, Fujii K, Otsuji S, Takiuchi S, Kakishita M, Ibuki M et al (2020) Optical coherence tomography characteristics of in-stent restenosis after drug-eluting stent implantation: a novel classification and its clinical significance. Heart Vessels 35:38–45
Acknowledgements
The authors thank the staffs in the catheterization laboratory at Red Cross Kyoto Daini Hospital for their excellent assistance during the study.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
This study was conducted in agreement with the Declaration of Helsinki and was approved by the institutional ethics committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Isodono, K., Fujii, K., Fujimoto, T. et al. The frequency and clinical characteristics of in-stent restenosis due to calcified nodule development after coronary stent implantation. Int J Cardiovasc Imaging 37, 15–23 (2021). https://doi.org/10.1007/s10554-020-01952-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-01952-z