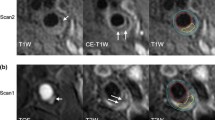
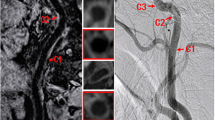
Abstract
The systemic nature of atherosclerotic disease may entail an association in disease severity between left and right carotid arteries. However, the etiology of plaque features in high-risk lesions is presumably attributed to local risk factors. We explored the symmetry of plaque morphology and composition across a broad range of atherosclerotic disease severities. All participants underwent carotid MR imaging on a 3.0 T scanner with a bilateral four-element phased-array surface coil. Vessel boundary and plaque components [calcification, lipid-rich necrotic core (LRNC) and intraplaque hemorrhage (IPH)] of bilateral carotid arteries were outlined. Normalized wall index (NWI) was calculated as follows: NWI = wall volume/total vessel volume. Carotid atherosclerosis score (CAS) was computed for plaque risk stratification. Associations of volume measurements between sides were evaluated using Pearson’s correlation. Cohen’s kappa was used to assess agreement between dichotomous variables. In the 177 participants with images of sufficient quality, there were very strong correlations between left and right lumen volumes (r = 0.85), total vessel volumes (r = 0.88), and strong correlations between wall volumes (r = 0.79), mean wall thickness (r = 0.66) and NWI (r = 0.71), and a moderate correlation between max wall thickness (r = 0.56) (all P < 0.001). There were moderate between-side agreements for the presence of calcification (κ = 0.54) and LRNC (κ = 0.49), but only fair agreement for IPH (κ = 0.31). The correlation of volume between left and right carotid arteries was strong for calcification (r = 0.62, P < 0.001) and weak for LRNC (r = 0.39, P < 0.001), but there was no significant correlation for IPH (r = 0.01, P = 0.99). Fair agreement (κ = 0.34) for CAS between paired carotid arteries was observed. Only 16 of 47 participants with CAS = 4 on at least one side had the same CAS on the contralateral side. Plaque morphology, calcification, and LRNC may develop symmetrically, but there is a relatively poor correlation for lipid content between sides. The weak symmetry of IPH and CAS indicates that the development of atherosclerosis into high-risk lesions may be regulated by local rather than systemic factors.




Similar content being viewed by others
Abbreviations
- NWI:
-
Normalized wall index
- CAS:
-
Carotid atherosclerosis score
- LRNC:
-
Lipid-rich necrotic core
- IPH:
-
Intraplaque hemorrhage
- IMT:
-
Intima-medial thickness
References
Heron M (2007) Deaths: leading causes for 2004. Natl Vital Stat Rep 56:1–95
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ et al (2014) Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129:e28–e292. doi:10.1161/01.cir.0000441139.02102.80
Hatsukami TS, Ferguson MS, Beach KW, Gordon D, Detmer P, Burns D, Alpers C, Strandness DE Jr (1997) Carotid plaque morphology and clinical events. Stroke 28:95–100. doi:10.1161/01.str.28.1.95
Zukowski AJ, Nicolaides AN, Lewis RT, Mansfield AO, Williams MA, Helmis E, Malouf GM, Thomas D, Al-Kutoubi A, Kyprianou P et al (1984) The correlation between carotid plaque ulceration and cerebral infarction seen on CT scan. J Vasc Surg 1:782–786. doi:10.1067/mva.1984.avs0010782
Devereux RB, Alderman MH (1993) Role of preclinical cardiovascular disease in the evolution from risk factor exposure to development of morbid events. Circulation 88:1444–1455. doi:10.1161/01.cir.88.4.1444
Vink A, Schoneveld AH, Richard W, de Kleijn DP, Falk E, Borst C, Pasterkamp G (2001) Plaque burden, arterial remodeling and plaque vulnerability: determined by systemic factors? J Am Coll Cardiol 38:718–723. doi:10.1016/s0735-1097(01)01444-9
Adams GJ, Simoni DM, Bordelon CB Jr, Vick GW 3rd, Kimball KT, Insull W Jr, Morrisett JD (2002) Bilateral symmetry of human carotid artery atherosclerosis. Stroke 33:2575–2580. doi:10.1161/01.str.0000035736.30488.7a
Howard G, Burke GL, Evans GW, Crouse JR 3rd, Riley W, Arnett D, de Lacy R, Heiss G (1994) Relations of intimal-medial thickness among sites within the carotid artery as evaluated by B-mode ultrasound. ARIC Investigators. Atherosclerosis Risk in Communities. Stroke 25:1581–1587. doi:10.1161/01.str.25.8.1581
Carallo C, Lucca LF, Ciamei M, Tucci S, de Franceschi MS (2006) Wall shear stress is lower in the carotid artery responsible for a unilateral ischemic stroke. Atherosclerosis 185:108–113. doi:10.1016/j.atherosclerosis.2005.05.019
Hansson GK, Libby P, Tabas I (2015) Inflammation and plaque vulnerability. J Intern Med 278:483–493. doi:10.1111/joim.12406
Levy AP, Moreno PR (2006) Intraplaque hemorrhage. Curr Mol Med 6:479–488. doi:10.2174/156652406778018626
Morrisett J, Vick W, Sharma R, Lawrie G, Reardon M, Ezell E, Schwartz J, Hunter G, Gorenstein D (2003) Discrimination of components in atherosclerotic plaques from human carotid endarterectomy specimens by magnetic resonance imaging ex vivo. Magn Reson Imaging 21:465–474. doi:10.1016/s0730-725x(02)00643-4
Chu B, Kampschulte A, Ferguson MS, Kerwin WS, Yarnykh VL, O’Brien KD, Polissar NL, Hatsukami TS, Yuan C (2004) Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke 35:1079–1084. doi:10.1161/01.str.0000125856.25309.86
Underhill HR, Yarnykh VL, Hatsukami TS, Wang J, Balu N, Hayes CE, Oikawa M, Yu W, Xu D, Chu B, Wyman BT, Polissar NL, Yuan C (2008) Carotid plaque morphology and composition: initial comparison between 1.5- and 3.0-T magnetic field strengths. Radiology 248:550–560. doi:10.1148/radiol.2482071114
Saam T, Yuan C, Chu B, Takaya N, Underhill H, Cai J, Tran N, Polissar NL, Neradilek B, Jarvik GP, Isaac C, Garden GA, Maravilla KR, Hashimoto B, Hatsukami TS (2007) Predictors of carotid atherosclerotic plaque progression as measured by noninvasive magnetic resonance imaging. Atherosclerosis 194:e34–e42. doi:10.1016/j.atherosclerosis.2006.08.016
Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, Hatsukami TS, Yuan C (2005) Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol 25:234–239. doi:10.1161/01.atv.0000149867.61851.31
Kerwin W, Xu D, Liu F, Saam T, Underhill H, Takaya N, Chu B, Hatsukami T, Yuan C (2007) Magnetic resonance imaging of carotid atherosclerosis: plaque analysis. Top Magn Reson Imaging 18:371–378. doi:10.1097/rmr.0b013e3181598d9d
Underhill HR, Hatsukami TS, Cai J, Yu W, DeMarco JK, Polissar NL, Ota H, Zhao X, Dong L, Oikawa M, Yuan C (2010) A noninvasive imaging approach to assess plaque severity: the carotid atherosclerosis score. Am J Neuroradiol 31:1068–1075. doi:10.3174/ajnr.a2007
Kundel HL, Polansky M (2003) Measurement of observer agreement. Radiology 228:303–308. doi:10.1148/radiol.2282011860
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174. doi:10.2307/2529310
Abedin M, Tintut Y, Demer LL (2004) Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 24:1161–1170. doi:10.1161/01.atv.0000133194.94939.42
Levin ME, Boisseau VC, Avioli LV (1976) Effects of diabetes mellitus on bone mass in juvenile and adult-onset diabetes. N Engl J Med 294:241–245. doi:10.1056/nejm197601292940502
Wissler RW (1991) Update on the pathogenesis of atherosclerosis. Am J Med 91:3S–9S. doi:10.1016/0002-9343(91)90050-8
Clarkson TB, Bond MG, Bullock BC, Marzetta CA (1981) A study of atherosclerosis regression in Macaca mulatta. IV. Changes in coronary arteries from animals with atherosclerosis induced for 19 months and then regressed for 24 or 48 months at plasma cholesterol concentrations of 300 or 200 mg/dl. Exp Mol Pathol 34:345–368. doi:10.1016/0014-4800(81)90052-6
Zhao XQ, Yuan C, Hatsukami TS, Frechette EH, Kang XJ, Maravilla KR, Brown BG (2001) Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: a case-control study. Arterioscler Thromb Vasc Biol 21:1623–1629. doi:10.1161/hq1001.098463
Helderman F, Segers D, de Crom R, Hierck BP, Poelmann RE, Evans PC, Krams R (2007) Effect of shear stress on vascular inflammation and plaque development. Curr Opin Lipidol 18:527–533. doi:10.1097/mol.0b013e3282ef7716
Sadat U, Weerakkody RA, Bowden DJ, Young VE, Graves MJ, Li ZY, Tang TY, Gaunt ME, Hayes PD, Gillard JH (2009) Utility of high resolution MR imaging to assess carotid plaque morphology: a comparison of acute symptomatic, recently symptomatic and asymptomatic patients with carotid artery disease. Atherosclerosis 207:434–439. doi:10.1016/j.atherosclerosis.2009.05.002
Cheung HM, Moody AR, Singh N, Bitar R, Zhan J, Leung G (2011) Late stage complicated atheroma in low-grade stenotic carotid disease: MR imaging depiction—prevalence and risk factors. Radiology 260:841–847. doi:10.1148/radiol.11101652
Qiao Y, Etesami M, Astor BC, Zeiler SR, Trout HH 3rd, Wasserman BA (2012) Carotid plaque neovascularization and hemorrhage detected by MR imaging are associated with recent cerebrovascular ischemic events. Am J Neuroradiol 33:755–760. doi:10.3174/ajnr.a2863
Takaya N, Yuan C, Chu B, Saam T, Polissar NL, Jarvik GP, Isaac C, McDonough J, Natiello C, Small R, Ferguson MS, Hatsukami TS (2005) Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation 111:2768–2775. doi:10.1161/circulationaha.104.504167
Xu D, Hippe DS, Underhill HR, Oikawa-Wakayama M, Dong L, Yamada K, Yuan C, Hatsukami TS (2014) Prediction of high-risk plaque development and plaque progression with the carotid atherosclerosis score. J Am Coll Cardiol Cardiovasc Imaging 7:366–373. doi:10.1016/j.jcmg.2013.09.022
Inzitari D, Eliasziw M, Gates P, Sharpe BL, Chan RK, Meldrum HE, Barnett HJ (2000) The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. N Engl J Med 342:1693–1700. doi:10.1056/nejm200006083422302
Saam T, Underhill HR, Chu B, Takaya N, Cai J, Polissar NL, Yuan C, Hatsukami TS (2008) Prevalence of American Heart Association type VI carotid atherosclerotic lesions identified by magnetic resonance imaging for different levels of stenosis as measured by duplex ultrasound. J Am Coll Cardiol 51:1014–1021. doi:10.1016/j.jacc.2007.10.054
Acknowledgments
We thank Xiaodong Zhang, Hongxia Sun, Rui Li and Wei Li for their technical assistance.
Author contribution
FL study design, data acquisition and analysis, manuscript drafting; XW study design, manuscript drafting and revising.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors Feiyu Li and Xiaoying Wang declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Research involving animal studies
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Li, F., Wang, X. Bilateral symmetry of human carotid artery atherosclerosis: a multi-contrast weighted MR study. Int J Cardiovasc Imaging 32, 1219–1226 (2016). https://doi.org/10.1007/s10554-016-0890-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-0890-4