Abstract
Purpose
To explore the effect of type 2 diabetes mellitus (T2DM) on the risk of death among women with breast cancer (BC).
Methods
A survival analysis was conducted among a cohort of women diagnosed with BC between 2006 and 2012 in Spain (n = 4,493). Biopsy or surgery confirmed BC cases were identified through the state population-based cancer registry with information on patients’ characteristics and vital status. Physician-diagnosed T2DM was confirmed based on primary health care clinical history. Cox regression analyses were used to estimate adjusted hazard ratios (aHR) and 95% confidence intervals (CI) for all-cause death. Analyses were adjusted for age, hospital size, several clinical characteristics (including BC stage and histology, among others) and treatment modalities.
Results
Among the 4,493 BC women, 388 (8.6%) had coexisting T2DM. Overall, 1,299 (28.9%) BC women died during the completion of the follow-up and 785 (17.5%) did so during the first five years after BC diagnosis, resulting in a five-year survival rate of 82.5%. The death rate was higher in women with T2DM (43.8% died during whole period and 26.0% during the first five years) when compared with women without T2DM (27.5% and 16.7%, respectively). Accordingly, all-cause mortality was higher in women with T2DM (aHR: 1.22; 95% CI 1.03–1.44), especially if T2DM was diagnosed before BC (aHR:1.24; 95% CI 1.03–1.50) and in women with BC diagnosed before 50 years (aHR: 2.38; 95% CI 1.04–5.48).
Conclusions
T2DM was associated with higher all-cause mortality among Spanish women with BC, particularly when the T2DM diagnosis was prior to the BC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most commonly diagnosed malignancy worldwide, even considering both sexes combined [1, 2]. After years of rising mortality rates, in the last decades BC survival has improved in most European countries, mainly due to advances in therapy and to the efficacy of screening programs for early diagnosis [3, 4]. In Spain, the five-year relative survival in 2000–07 was 82.8%, which meant an increase of 2.4 percent concerning the last decade of the twentieth century [5]. Likewise, Clèries et al. [6] found a 19 percent reduction in the 10-year risk of death of women diagnosed with BC in 1995–2004 compared to women diagnosed in 1985–1994. Moreover, according to current estimates on mortality by the International Agency for Research on Cancer, Spain is the European country with the lowest age-standardized BC death rates [2]. Nevertheless, there is still room for improvement in BC survival.
There is a growing body of evidence linking type 2 diabetes mellitus (T2DM) and BC. Both are diseases associated with aging that share a wide variety of risk factors, including socioeconomic conditions, lifestyle behaviors, and body fat [7, 8]. Moreover, T2DM and BC have been hypothesized to be causally associated through the tumorigenic effect of hyperinsulinemia, insulin-like growth factors, and other hormones [9, 10]. Several meta-analyzes have found a pooled 15–20% excess of risk of BC among women with preexisting T2DM [11,12,13], even adjusting for overweight/obesity, which is the main shared risk factor. Specifically, a preexisting T2DM has been found to increase the risk of BC with poor prognosis [14]. Accordingly, there is also strong evidence from studies involving different countries and races about the increased risk of death among women with BC and preexisting T2DM, when compared with women with BC, but who are T2DM-free [15,16,17,18]. Nevertheless, the impact of T2DM on mortality among women with BC has not been adequately studied in Spain. Indeed, the only study identified was published in 2019 and, although some findings were reported about the association between T2DM and BC, the study was not specifically focused on these factors. In a cohort of 7.338 women with BC diagnosed from 1985–2004 in Girona (Spain), Ameijide et al. [19] found a 1.43 standardized mortality ratio among women with T2DM, after a 10-year follow-up.
Therefore, to examine the consistency of these results and determine the effect of the timing of the T2DM diagnosis, a study was conducted to explore the effect of T2DM (preexisting or subsequent) on the risk of death among women with BC.
Subjects and methods
Study design and participants
A survival analysis was conducted among a cohort of women diagnosed with BC between 2006 and 2012 in Asturias (Spain) and retrospectively followed-up until the end of 2019. Data were obtained from the state population-based cancer registry, which is one of the oldest established cancer registries in Spain, and which collaborates with the International Agency for Research on Cancer by cancer data provision.
An anonymous database was created in STATA v.15 (StataCorp, College Station, Texas), with units working with biopsy or surgery confirmed BC cases (n = 4,704 cases, after excluding 56 cases of BC among males). Subsequently, to avoid any overestimation due to multiple BC on survival, a single case of BC was selected per woman, by excluding all but one case of BC diagnosed in the same woman. When a woman had two or more diagnoses of BC, the first diagnosed case of BC was selected if both cases were metachronous (i.e., time between diagnoses > 6 months), or, the case with the worse prognosis was selected (i.e. more advanced stage and/or greater treatment intensity) if they were synchronous.
The study protocol was approved by the Research Ethics Committee of Asturias (Spain). The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its subsequent amendments.
T2DM and mortality ascertainment
First, we merged the database from the cancer registry with the computerized clinical history of primary healthcare (OMI-AP software) to identify BC women with an additional physician-diagnosed T2DM. Subsequently, we performed a computerized search of the National Death Index to evaluate all-cause mortality. This database contains updated information on the vital status of all residents in Spain. Information regarding death date after 2006 was available for 99.9% of the cohort. Censoring was set at the date of death or at the end of follow-up (31 December 2019), whichever occurred first.
Covariates
Covariates were selected by combining two criteria. On the one hand, these should be variables linked to either T2DM or mortality according to scientific literature. On the other hand, they should be variables for which information is available on the administrative databases used in this study (i.e., cancer registry, OMI-AP software and National Death Index) and with the possibility of automatic data extraction. Given that some important predictors of death, such as body weight and comorbid conditions, were only available in plain text on health records (and not stated in all records), their inclusion would have implied both an exhaustive manual review and a reduction of the study population. Therefore, this information was not considered in the study.
The study variables included age at BC and T2DM diagnosis, hospital size, measured by number of beds (< 500, ≥ 500), BC suspected via a population screening program (yes/no), another non-BC cancer (yes/no), BC staging and histology were coded according to the American Joint Committee on Cancer and the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) [20, 21], BC location (breast quadrants, central part of the breast, nipple, axillary extensions, contiguous sites, unspecified sites) and types of treatments, including surgery, chemotherapy, radiotherapy, hormone therapy, immunotherapy, targeted therapy, among others.
Data analysis
Of the 4,704 BC cases included in the study, 37 were excluded because they were duplicates, suggesting coding errors, and 164 were excluded because they were multiple BC cases (27 metachronous and 137 synchronous). Of the remaining 4,503 women with BC, 10 were excluded due to missing information on the national identity document, and consequently, a lack of information on survival status. Finally, the analyses were conducted in 4,493 women with BC.
Cox proportional-hazards models were used to assess the association between T2DM and mortality during the first five years of follow-up and mortality throughout the entire period among women with BC (mean years of follow-up was 8.66). Additionally, to study the effect of the time of T2DM diagnosis on survival, we performed two analyses with T2DM, classified into categories. The first one used two categories (diagnosis of T2DM before or after BC) whereas the second used three categories (diagnosis of T2DM > 5 years before BC, 0–5 years before BC and after BC). Women without T2DM were always used as the reference. In all cases, we built two Cox models, the first one was crude, whereas the second one was adjusted. Previously, a bivariate analysis was used to identify the variables related to both T2DM and death, to consider those with a p-value < 0.2 as potential confounders in the adjusted model. The variables included in the adjusted model were age at BC diagnosis (< 50, 50–59, 60–69, ≥ 70 years), hospital size (< 500, ≥ 500 beds), BC suspected via screening (yes/no), other non-BC cancer (yes/no), BC stage (0-I, II, III-IV, not applicable, unknown), BC histology (non-invasive ductal carcinoma, invasive ductal carcinoma, non-invasive lobular carcinoma, invasive lobular carcinoma, other ductal and lobular, other types), BC localization (any breast quadrant, central part/nipple, axillary/contiguous sites, unspecified site) and types of BC treatments, including surgery, chemotherapy, radiotherapy, hormone therapy, immunotherapy, targeted therapy and others. Given that there were many possible combinations, BC treatment modalities were used as dichotomous variables for data analysis. Finally, we used the Kaplan–Meier method to estimate the unadjusted survival function and the log-rank test to compare survival curves. Statistical significance was set at p < 0.05.
Results
Among the cohort of women with BC (n = 4,493), 388 (8.6%) had a physician-diagnosed T2DM. In three out of four women (75.3%) T2DM was diagnosed before BC. Statistically significant differences between women with and without T2DM were only found for age of BC and treatment types. Compared to women without T2DM, those with T2DM were older (p < 0.001) and had lower intensity treatment, as they received more hormone therapy (p = 0.018), although less surgery (p = 0.001), chemotherapy (p < 0.001), radiotherapy (p = 0.023) and targeted therapy (p = 0.003) (Table 1).
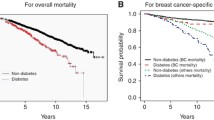
Overall, 1,299 (28.9%) women with BC died during complete follow-up and 785 (17.5%) did so during the first five years after BC diagnosis; therefore, the five-year survival rate was 82.5%. Compared to women without T2DM, the death rate was 59.3% higher in women with T2DM during the entire follow-up period (27.5% vs. 43.8%; p < 0.001) and 55.7% during the first 5 years (16.7% vs. 26.0%; p < 0.001). Crude survival curves according to T2DM diagnosis are shown in Fig. 1.
Women with coexisting BC and T2DM had a higher mortality risk when compared with those without T2DM: the adjusted hazard ratio (aHR) for overall mortality was 1.22 (95% CI 1.03–1.44) and 1.21 for five-year mortality (95% CI 0.98–1.51) (Table 2). When women were classified depending on whether the T2DM had been diagnosed before or after BC, a statistically significant mortality risk was only found among women with T2DM diagnosed before BC (aHR: 1.24; 95% CI 1.03–1.50 for overall mortality, and aHR: 1.26; 95% CI 1.00–1.60 for five-year mortality) (Table 3). Furthermore, in the analysis using three categories according to the time of T2DM diagnosis, the mortality risk was restricted to women with T2DM diagnosed > 5 years before BC (aHR: 1.31; 95%CI 1.01–1.69 for overall mortality, and aHR: 1.37; 95% CI 1.00–1.91 for five-year mortality) (Supplementary Table S1). In the ancillary analysis, we found that the excess of mortality risk associated with T2DM was only among women aged < 70 years old at BC diagnosis (Table 4).
Discussion
This survival analysis conducted among a cohort of 4,493 Spanish women with BC, showed that coexisting T2DM was associated with higher mortality after a mean of 8.66 years of follow-up. Moreover, a higher risk of mortality was seen among women with a longer history of T2DM (diagnosed > 5 years before BC) and in those with a younger age at BC diagnosis.
The five-year overall survival rate after BC in our cohort (82.5%) was consistent with findings from other studies involving Spanish populations. Chirlaque et al., using data from nine population-based registries, reported a five-year survival rate of 82.8% among women diagnosed between 2000 and 2007 [5]. Similarly, Baeyens-Fernández et al., found a five-year survival rate in Granada (Spain) of 83.7% in a cohort of women with a BC diagnosed in 2010–2012 (n = 8.502) [22]. Nevertheless, the overall survival rate after BC was lower than Northern European countries, the USA or Australia [23, 24], probably due to a socioeconomic gap in favor of these countries, which conditions more innovative treatment options, better established screening programs and more advanced diagnoses [25]. The lower survival rate in Spain is important to understand our findings related to the lower survival of Spanish women with BC and T2DM when compared with other studies. For instance, Maskarinec et al., in a survival analysis of a multiethnic cohort derived from cancer registries in Hawaii and California, found that 79.1% of women with T2DM were alive five years after BC diagnosis [17], representing a survival rate of approximately 5 percent higher than our cohort. Nevertheless, given that the study by Maskarinec et al., excluded women under 45 years of age at BC diagnosis [17], which is the age group with higher mortality rates after BC, according to our results, their survival rate may have been overestimated.
Although we found lower survival rates in our cohort after BC both for women with and without T2DM, the magnitude of the association between T2DM status and the risk of death could have been similar to other studies. However, the risk of death in women with BC associated to T2DM status was lower than reported in most studies, suggesting that T2DM could be a less important predictor of death for women with BC in Spain compared to other countries. In 2016, Zhao and Ren published a meta-analysis of 17 studies involving 48,315 women with BC from North America, Europe, and Asia [13]. The pooled adjusted HR of all-cause death was 1.51 (95% CI 1.34–1.70) and 1.46 (95% CI 1.21–1.76) when analyses were restricted to the eight studies with follow-up > 5 years, as in our study. More recently, Baglia et al., using data from participants of two population-based cohort studies in Shanghai, found an adjusted HR of 1.56 (95% CI 1.01–2.43) for all-cause death over a median follow-up of 3.4 years after BC diagnosis [26].
As in previous studies, we observed that the excess risk of mortality among women with BC and T2DM was dependent on the duration of T2DM. Thereby, the elevated all-cause mortality was only found for BC patients with longer T2DM duration. This finding may be attributed to the deleterious effect of common long-term T2DM complications. Luo et al., [27] and Maskarinec et al., [17], using a cut-off point of 7 years to define long T2DM duration, found a HR for overall mortality of 1.32 (95% CI 1.06, 1.66) and 1.27 (95% CI 1.07–1.49), respectively, which are almost equal to our figures. Moreover, Lega et al., [28] reported a HR of 1.28 (95% 1.15–1.43) using the same cut-off point for T2DM duration as in our study (five years), however, their analysis only involved women with stage III BC.
The higher mortality of women with BC and T2MD compared to women with only BC may be due to the long-term health effects of T2DM, that include deaths related to renal complications, and cardiovascular and cerebral vascular diseases [29, 30]. In addition, the interaction of BC and T2DM could be based on certain biological mechanisms that may help explain the effect of T2DM on survival of women with BC. Tumor development usually produces chronic inflammation, an increase of insulin growth factor and hyperinsulinemia [31, 32], which can lead to BC with worse prognosis [33]. Nevertheless, given that there were no differences in BC characteristics between women with or without T2DM from our cohort -even stage 0-I was more frequent among women with T2DM- the higher risk of death in women with T2DM was probably due to health conditions related to T2DM rather than to the effects of BC decreasing survival. Although we have no information about the cause of death, the majority of studies found that T2DM greatly increased the risk of death from other causes, and to a lower extent (or had no effect on) the risk of death for BC [17, 27, 28, 34, 35]. Moreover, this interpretation is coherent with the finding related to longer duration of T2DM, which may be an indicator not only of higher probability of vascular complications in damaged organs, but also of greater disease severity, higher accumulation of antidiabetic treatments and increased probability of uncontrolled T2DM. Consequently, it should be recognized that adjusting for types of T2DM treatment and glucose control could have changed our findings, as it would have enabled us to classify women according to disease severity and therapeutic management. Another possible explanation for the higher risk of mortality among women with T2DM lies in the differences in BC treatment in our data series. Compared to women without T2DM, those with T2DM were treated with significantly less intensity, which could lead to lower survival rates. It cannot be ruled out that women with preexisting T2DM, and thus with compromised cardiometabolic health, may have received lower BC treatment intensity due to their worse baseline health status. In any case, the differences in therapeutic approaches of BC according to T2DM status and their potential effect on survival merits further research.
Finally, the inverse dose–response effect of the age at BC diagnosis on the association between T2DM and the risk of death could be conditioned by several factors. First, young women with T2DM and BC usually have tumors with worse prognosis [14, 36]. Second, they could also have more treatment side effects and complications, especially related to BC surgery [37]. Third, there is a growing body of evidence suggesting that young-onset T2DM has worse glycemic and lipid control, and more complications compared to older onset T2DM patients [38]. In any case, this finding underscores the need for a rigorous follow-up of BC complications and a more exhaustive metabolic control of T2DM among young women.
Our study has some important limitations that need to be considered when interpreting the findings. First, we lack information on anthropometric measures, which are well stablished predictors of adverse health outcomes and death both among individuals with BC and T2DM. Particularly, we have no measure of body fat, despite the fact that endocrine alternations caused by excessive adipose tissue can induce tumor progression, leading to a decrease in life expectancy. Moreover, weight gain is also common after BC treatments, with a consequent potential impact on T2DM control. In short, consideration should be given to the close relation of obesity with the T2DM/BC binomial, as adjusting for body weight would probably reduce or eliminate statistically significant differences in death rates between BC women with or without T2DM. Second, we also lack data on socioeconomic characteristics, lifestyle variables and comorbidity, although it is known that certain unhealthy behaviors, low education level and comorbid conditions commonly associated to T2DM may lead to poor overall health status. Thereby, we cannot disentangle the effects of T2DM related factors from the disease itself. Third, although we used a public administrative database that is very accurate to ascertain date of death, this database contains no information about the specific cause of death. Thus, our study was unable to determine whether T2DM increased the risk of death for BC or for other causes. Nevertheless, misclassification when assigning cause of death may be highly relevant when studying women with BC and T2DM. Patients who die shortly after BC diagnosis may be more likely to be assigned BC as cause of death, whereas women dying after BC remission may be more prone to be classified as having a cardiovascular cause of death, because of the well-known association of T2DM with cardiovascular mortality.
In conclusion, Spanish women with T2DM prior to BC had worse all-cause survival, particularly when BC was diagnosed before the age of 50. Our findings add consistency to the previous studies among BC women from several countries and ethnic groups. Nevertheless, considering the lack of information regarding certain relevant variables, including body weight, morbidity and T2DM treatment, caution is suggested when interpreting the study findings. Longitudinal research is needed to study whether the clinical and psychological impact of BC diagnosis disrupts T2DM management, affecting health status and decreasing survival rates.
Data availability
Public data from the state population-based cancer registry of Asturias (Spain) are available on reasonable request.
Code availability
Not applicable.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Ferlay J, Ervik M, Lam F et al (2020). Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer. https://gco.iarc.fr/today. Accessed 26 June 2021.
Carioli G, Malvezzi M, Rodriguez T et al (2017) Trends and predictions to 2020 in breast cancer mortality in Europe. Breast 36:89–95. https://doi.org/10.1016/j.breast.2017.06.003
Crocetti E, Roche L, Buzzoni C et al (2017) Trends in net survival from breast cancer in six European Latin countries: results from the SUDCAN population-based study. Eur J Cancer Prev 26:S85–S91. https://doi.org/10.1097/CEJ.0000000000000291
Chirlaque MD, Salmerón D, Galceran J et al (2018) Cancer survival in adult patients in Spain. Results from nine population-based cancer registries. Clin Transl Oncol 20:201–211. https://doi.org/10.1007/s12094-017-1710-6
Clèries R, Ameijide A, Buxó M et al (2018) Long-term crude probabilities of death among breast cancer patients by age and stage: a population-based survival study in Northeastern Spain (Girona-Tarragona 1985–2004). Clin Transl Oncol 20:1252–1260. https://doi.org/10.1007/s12094-018-1852-1
Eyre H, Kahn R, Robertson RM; American Cancer Society, the American Diabetes Association, and the American Heart Association. Collaborative Writing Committee (2004) Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Diabetes Care 27:1812–1824. https://doi.org/10.2337/diacare.27.7.1812
Clinton SK, Giovannucci EL, Hursting SD (2020) The world cancer research fund/American institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr 150:663–671. https://doi.org/10.1093/jn/nxz268
Johnson JA, Carstensen B, Witte D et al (2012) Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia 55:1607–1618. https://doi.org/10.1007/s00125-012-2525-1
Kang C, LeRoith D, Gallagher EJ (2018) Diabetes, obesity, and breast cancer. Endocrinology 159:3801–3812. https://doi.org/10.1210/en.2018-00574
Boyle P, Boniol M, Koechlin A et al (2012) Diabetes and breast cancer risk: a meta-analysis. Br J Cancer 107:1608–1617. https://doi.org/10.1038/bjc.2012.414
Tsilidis KK, Kasimis JC, Lopez DS et al (2015) Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 350:g7607. https://doi.org/10.1136/bmj.g7607
Zhao XB, Ren GS (2016) Diabetes mellitus and prognosis in women with breast cancer: a systematic review and meta-analysis. Medicine (Baltimore) 95:e5602. https://doi.org/10.1097/MD.0000000000005602
García-Esquinas E, Guinó E, Castaño-Vinyals G et al (2016) Association of diabetes and diabetes treatment with incidence of breast cancer. Acta Diabetol 53(1):99–107. https://doi.org/10.1007/s00592-015-0756-6
Luo J, Hendryx M, Virnig B et al (2015) Pre-existing diabetes and breast cancer prognosis among elderly women. Br J Cancer 113:827–832. https://doi.org/10.1038/bjc.2015.249
Chen Y, Wu F, Saito E et al (2017) Association between type 2 diabetes and risk of cancer mortality: a pooled analysis of over 771,000 individuals in the Asia Cohort Consortium. Diabetologia 60:1022–1032. https://doi.org/10.1007/s00125-017-4229-z
Maskarinec G, Shvetsov YB, Conroy SM et al (2019) Type 2 diabetes as a predictor of survival among breast cancer patients: the multiethnic cohort. Breast Cancer Res Treat 173:637–645. https://doi.org/10.1007/s10549-018-5025-2
Charlot M, Castro-Webb N, Bethea TN et al (2017) Diabetes and breast cancer mortality in Black women. Cancer Causes Control 28:61–67. https://doi.org/10.1007/s10552-016-0837-z
Ameijide A, Clèries R, Carulla M et al (2019) Cause-specific mortality after a breast cancer diagnosis: a cohort study of 10,195 women in Girona and Tarragona. Clin Transl Oncol 21:1014–1025. https://doi.org/10.1007/s12094-018-02015-5
Giuliano AE, Connolly JL, Edge SB et al. (2017) Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67(4): 290‐303 Doi: https://doi.org/10.3322/caac.21393
Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S (eds) (2000) International Classification of Diseases for Oncology, 3rd edn. World Health Organization, Geneva
Baeyens-Fernández JA, Molina-Portillo E, Pollán M et al (2018) Trends in incidence, mortality and survival in women with breast cancer from 1985 to 2012 in Granada, Spain: a population-based study. BMC Cancer 18:781. https://doi.org/10.1186/s12885-018-4682-1
Sant M, Chirlaque Lopez MD, Agresti R et al (2015) Survival of women with cancers of breast and genital organs in Europe 1999–2007: Results of the EUROCARE-5 study. Eur J Cancer 51:2191–2205. https://doi.org/10.1016/j.ejca.2015.07.022
Allemani C, Matsuda T, Di Carlo V et al (2018) Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391:1023–1075. https://doi.org/10.1016/S0140-6736(17)33326-3
Wojtyla C, Bertuccio P, Wojtyla A et al (2021) European trends in breast cancer mortality, 1980–2017 and predictions to 2025. Eur J Cancer 152:4–17. https://doi.org/10.1016/j.ejca.2021.04.026
Baglia ML, Cui Y, Zheng T et al (2019) Diabetes medication use in association with survival among patients of breast, colorectal, lung, or gastric cancer. Cancer Res Treat 51:538–546. https://doi.org/10.4143/crt.2017.591
Luo J, Virnig B, Hendryx M et al (2014) Diabetes, diabetes treatment and breast cancer prognosis. Breast Cancer Res Treat 148:153–162. https://doi.org/10.1007/s10549-014-3146-9
Lega IC, Austin PC, Fischer HD et al (2018) The impact of diabetes on breast cancer treatments and outcomes: a population-based study. Diabetes Care 41:755–761. https://doi.org/10.2337/dc17-2012
Raghavan S, Vassy JL, Ho YL et al (2019) Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J Am Heart Assoc 8:e011295. https://doi.org/10.1161/JAHA.118.011295
Ling W, Huang Y, Huang YM et al (2020) Global trend of diabetes mortality attributed to vascular complications, 2000–2016. Cardiovasc Diabetol 19:182. https://doi.org/10.1186/s12933-020-01159-5
Godsland IF (2009) Insulin resistance and hyperinsulinaemia in the development and progression of cancer. Clin Sci (Lond) 118:315–332. https://doi.org/10.1042/CS20090399
Novosyadlyy R, Lann DE, Vijayakumar A et al (2010) Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res 70:741–751. https://doi.org/10.1158/0008-5472.CAN-09-2141
Lipscombe LL, Fischer HD, Austin PC et al (2015) The association between diabetes and breast cancer stage at diagnosis: a population-based study. Breast Cancer Res Treat 150:613–620. https://doi.org/10.1007/s10549-015-3323-5
Patnaik JL, Byers T, DiGuiseppi C et al (2011) Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res 13:R64. https://doi.org/10.1186/bcr2901
Cleveland RJ, North KE, Stevens J et al (2012) The association of diabetes with breast cancer incidence and mortality in the Long Island Breast Cancer Study Project. Cancer Causes Control 23:1193–1203. https://doi.org/10.1007/s10552-012-9989-7
Bronsveld HK, Jensen V, Vahl P et al (2017) Diabetes and breast cancer subtypes. PLoS ONE 12:e0170084. https://doi.org/10.1371/journal.pone.0170084
Lopez-de-Andres A, Jimenez-Trujillo I, Hernandez-Barrera V et al (2017) Association of type 2 diabetes with in-hospital complications among women undergoing breast cancer surgical procedures. A retrospective study using the Spanish National Hospital Discharge Database, 2013–2014. BMJ Open 7:e017676. https://doi.org/10.1136/bmjopen-2017-017676
Magliano DJ, Sacre JW, Harding JL et al (2020) Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nat Rev Endocrinol 16:321–331. https://doi.org/10.1038/s41574-020-0334-z
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by a public grant from the University of Oviedo (Ref. PAPI-18-EMERG-5). The funding agency had no role in the study design, data analysis, interpretation of results, writing of the report, and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
LF-A and AL conceived and designed the study. NR-R and AL conducted the statistical analyses. LF-A and AL drafted the first version of the manuscript, which was revised by AF-F, ALL-F and AIE-M. Thereby, all authors made substantial contributions to the analysis and interpretation of the data, revised the manuscript for important intellectual content, and approved the final version. AF-F and AL are guarantors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernández-Arce, L., Robles-Rodríguez, N., Fernández-Feito, A. et al. Type 2 Diabetes and all-cause mortality among Spanish women with breast cancer. Cancer Causes Control 33, 271–278 (2022). https://doi.org/10.1007/s10552-021-01526-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-021-01526-x