Abstract
Purpose
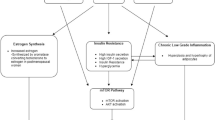
Positive energy imbalance and growth factors linked to obesity promote the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin (mTOR) pathway. As the obesity–breast cancer associations differ between European American (EA) and African-American (AA) women, we investigated genetic variants in the mTOR pathway and breast cancer risk in these two racial groups.
Methods
We examined 400 single-nucleotide polymorphisms (SNPs) in 31 mTOR pathway genes in the Women’s Circle of Health Study with 1263 incident breast cancers (645 EA, 618 AA) and 1382 controls (641 EA, 741 AA). Multivariable logistic regression was performed separately within racial groups. Effect modification was assessed for measured body size and weight gain since age 20.
Results
In EA women, variants in FRAP1 rs12125777 (intron), PRR5L rs3740958 (synonymous coding), and CDKAL1 rs9368197 (intron) were associated with increased breast cancer risk, while variants in RPTOR rs9900506 (intron) were associated with decreased risk (nominal p-trend for functional and FRAP1 SNPs or p adjusted for correlated test [p ACT] < 0.05). For AA women, variants in RPTOR rs3817293 (intron), PIK3R1 rs7713645 (intron), and CDKAL1 rs9368197 were associated with decreased breast cancer risk. The significance for FRAP1 rs12125777 and RPTOR rs9900506 in EA women did not hold after correction for multiple comparisons. The risk associated with FRAP1 rs12125777 was higher among EAs who had body mass index ≥30 kg/m2 (odds ratio = 7.69, 95 % CI 2.11–28.0; p-interaction = 0.007) and gained weight ≥35 lb since age 20 (odds ratio = 3.34, 95 % CI 1.42–7.85; p-interaction = 0.021), compared to their counterparts.
Conclusions
The mTOR pathway may be involved in breast cancer carcinogenesis differently for EA and AA women.

Similar content being viewed by others
References
World Cancer Research Fund/American Institute for Cancer Research (2010) Continuous update project report. Food, Nutrition, Physical Activity, and the Prevention of Breast Cancer
Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A (2014) Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev 36(1):114–136. doi:10.1093/epirev/mxt010
Bandera EV, Chandran U, Zirpoli G, Gong Z, McCann SE, Hong CC, Ciupak G, Pawlish K, Ambrosone CB (2013) Body fatness and breast cancer risk in women of African ancestry. BMC Cancer 13:475. doi:10.1186/1471-2407-13-475
Bandera EV, Chandran U, Hong CC, Troester MA, Bethea TN, Adams-Campbell LL, Haiman CA, Park SY, Olshan AF, Ambrosone CB, Palmer JR, Rosenberg L (2015) Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat 150(3):655–666. doi:10.1007/s10549-015-3353-z
White AJ, Nichols HB, Bradshaw PT, Sandler DP (2015) Overall and central adiposity and breast cancer risk in the sister study. Cancer. doi:10.1002/cncr.29552
Harvie M, Hooper L, Howell AH (2003) Central obesity and breast cancer risk: a systematic review. Obes Rev 4(3):157–173
Amadou A, Ferrari P, Muwonge R, Moskal A, Biessy C, Romieu I, Hainaut P (2013) Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev 14(8):665–678. doi:10.1111/obr.12028
Vucenik I, Stains JP (2012) Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci 1271:37–43. doi:10.1111/j.1749-6632.2012.06750.x
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12(1):21–35. doi:10.1038/nrm3025
Hall KD, Heymsfield SB, Kemnitz JW, Klein S, Schoeller DA, Speakman JR (2012) Energy balance and its components: implications for body weight regulation. Am J Clin Nutr 95(4):989–994. doi:10.3945/ajcn.112.036350
Rugo HS, Keck S (2012) Reversing hormone resistance: have we found the golden key? J Clin Oncol 30(22):2707–2709. doi:10.1200/JCO.2012.42.1271
Martin LA, Andre F, Campone M, Bachelot T, Jerusalem G (2013) mTOR inhibitors in advanced breast cancer: ready for prime time? Cancer Treat Rev 39(7):742–752. doi:10.1016/j.ctrv.2013.02.005
De Araujo ME, Erhart G, Buck K, Muller-Holzner E, Hubalek M, Fiegl H, Campa D, Canzian F, Eilber U, Chang-Claude J, Coassin S, Haun M, Kedenko L, Paulweber B, Reitsamer R, Himmel I, Flesch-Janys D, Lamina C, Kronenberg F, Huber LA, Kloss-Brandstatter A (2013) Polymorphisms in the gene regions of the adaptor complex LAMTOR2/LAMTOR3 and their association with breast cancer risk. PLoS ONE 8(1):e53768. doi:10.1371/journal.pone.0053768
Mehta MS, Vazquez A, Kulkarni DA, Kerrigan JE, Atwal G, Metsugi S, Toppmeyer DL, Levine AJ, Hirshfield KM (2011) Polymorphic variants in TSC1 and TSC2 and their association with breast cancer phenotypes. Breast Cancer Res Treat 125(3):861–868. doi:10.1007/s10549-010-1062-1
Shu X, Lin J, Wood CG, Tannir NM, Wu X (2013) Energy balance, polymorphisms in the mTOR pathway, and renal cell carcinoma risk. J Natl Cancer Inst 105(6):424–432. doi:10.1093/jnci/djt005
Lin J, Wang J, Greisinger AJ, Grossman HB, Forman MR, Dinney CP, Hawk ET, Wu X (2010) Energy balance, the PI3K-AKT-mTOR pathway genes, and the risk of bladder cancer. Cancer Prev Res (Phila) 3(4):505–517. doi:10.1158/1940-6207.CAPR-09-0263
Ambrosone CB, Ciupak GL, Bandera EV, Jandorf L, Bovbjerg DH, Zirpoli G, Pawlish K, Godbold J, Furberg H, Fatone A, Valdimarsdottir H, Yao S, Li Y, Hwang H, Davis W, Roberts M, Sucheston L, Demissie K, Amend KL, Tartter P, Reilly J, Pace BW, Rohan T, Sparano J, Raptis G, Castaldi M, Estabrook A, Feldman S, Weltz C, Kemeny M (2009) Conducting molecular epidemiological research in the age of HIPAA: a multi-institutional case–control study of breast cancer in African-American and European-American women. J Oncol 2009:871250. doi:10.1155/2009/871250
Bandera EV, Chandran U, Zirpoli G, McCann SE, Ciupak G, Ambrosone CB (2013) Rethinking sources of representative controls for the conduct of case–control studies in minority populations. BMC Med Res Methodol 13:71. doi:10.1186/1471-2288-13-71
Chang CL, Cai JJ, Cheng PJ, Chueh HY, Hsu SY (2011) Identification of metabolic modifiers that underlie phenotypic variations in energy-balance regulation. Diabetes 60(3):726–734. doi:10.2337/db10-1331
Xu Z, Taylor JA (2009) SNPinfo: integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res 37(Web Server issue):W600–W605. doi:10.1093/nar/gkp290
International HapMap Consortium, Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, Belmont JW, Boudreau A, Hardenbol P, Leal SM, Pasternak S, Wheeler DA, Willis TD, Yu F, Yang H, Zeng C, Gao Y, Hu H, Hu W, Li C, Lin W, Liu S, Pan H, Tang X, Wang J, Wang W, Yu J, Zhang B, Zhang Q, Zhao H, Zhou J, Gabriel SB, Barry R, Blumenstiel B, Camargo A, Defelice M, Faggart M, Goyette M, Gupta S, Moore J, Nguyen H, Onofrio RC, Parkin M, Roy J, Stahl E, Winchester E, Ziaugra L, Altshuler D, Shen Y, Yao Z, Huang W, Chu X, He Y, Jin L, Liu Y, Sun W, Wang H, Wang Y, Xiong X, Xu L, Waye MM, Tsui SK, Xue H, Wong JT, Galver LM, Fan JB, Gunderson K, Murray SS, Oliphant AR, Chee MS, Montpetit A, Chagnon F, Ferretti V, Leboeuf M, Olivier JF, Phillips MS, Roumy S, Sallee C, Verner A, Hudson TJ, Kwok PY, Cai D, Koboldt DC, Miller RD, Pawlikowska L, Taillon-Miller P, Xiao M, Tsui LC, Mak W, Song YQ, Tam PK, Nakamura Y, Kawaguchi T, Kitamoto T, Morizono T, Nagashima A, Ohnishi Y, Sekine A, Tanaka T, Tsunoda T, Deloukas P, Bird CP, Delgado M, Dermitzakis ET, Gwilliam R, Hunt S, Morrison J, Powell D, Stranger BE, Whittaker P, Bentley DR, Daly MJ, de Bakker PI, Barrett J, Chretien YR, Maller J, McCarroll S, Patterson N, Pe’er I, Price A, Purcell S, Richter DJ, Sabeti P, Saxena R, Schaffner SF, Sham PC, Varilly P, Stein LD, Krishnan L, Smith AV, Tello-Ruiz MK, Thorisson GA, Chakravarti A, Chen PE, Cutler DJ, Kashuk CS, Lin S, Abecasis GR, Guan W, Li Y, Munro HM, Qin ZS, Thomas DJ, McVean G, Auton A, Bottolo L, Cardin N, Eyheramendy S, Freeman C, Marchini J, Myers S, Spencer C, Stephens M, Donnelly P, Cardon LR, Clarke G, Evans DM, Morris AP, Weir BS, Mullikin JC, Sherry ST, Feolo M, Skol A, Zhang H, Matsuda I, Fukushima Y, Macer DR, Suda E, Rotimi CN, Adebamowo CA, Ajayi I, Aniagwu T, Marshall PA, Nkwodimmah C, Royal CD, Leppert MF, Dixon M, Peiffer A, Qiu R, Kent A, Kato K, Niikawa N, Adewole IF, Knoppers BM, Foster MW, Clayton EW, Watkin J, Muzny D, Nazareth L, Sodergren E, Weinstock GM, Yakub I, Birren BW, Wilson RK, Fulton LL, Rogers J, Burton J, Carter NP, Clee CM, Griffiths M, Jones MC, McLay K, Plumb RW, Ross MT, Sims SK, Willey DL, Chen Z, Han H, Kang L, Godbout M, Wallenburg JC, L’Archeveque P, Bellemare G, Saeki K, An D, Fu H, Li Q, Wang Z, Wang R, Holden AL, Brooks LD, McEwen JE, Guyer MS, Wang VO, Peterson JL, Shi M, Spiegel J, Sung LM, Zacharia LF, Collins FS, Kennedy K, Jamieson R, Stewart J (2007) A second generation human haplotype map of over 3.1 million SNPs. Nature 449 (7164):851–861. doi:10.1038/nature06258
Ruiz-Narvaez EA, Rosenberg L, Wise LA, Reich D, Palmer JR (2011) Validation of a small set of ancestral informative markers for control of population admixture in African Americans. Am J Epidemiol 173(5):587–592. doi:10.1093/aje/kwq401
Pritchard JK, Stephens M, Rosenberg NA, Donnelly P (2000) Association mapping in structured populations. Am J Hum Genet 67(1):170–181. doi:10.1086/302959
Conneely KN, Boehnke M (2007) So many correlated tests, so little time! Rapid adjustment of P values for multiple correlated tests. Am J Hum Genet 81(6):1158–1168. doi:10.1086/522036
Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, Karczewski KJ, Park J, Hitz BC, Weng S, Cherry JM, Snyder M (2012) Annotation of functional variation in personal genomes using RegulomeDB. Genome Res 22(9):1790–1797. doi:10.1101/gr.137323.112
World Health Organization (2011) Waist circumference and waist–hip ratio. Report of a WHO expert consultation, Geneva, 8–11 December 2008
Encode Project Consortium (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489(7414):57–74. doi:10.1038/nature11247
Baynes KC, Beeton CA, Panayotou G, Stein R, Soos M, Hansen T, Simpson H, O’Rahilly S, Shepherd PR, Whitehead JP (2000) Natural variants of human p85 alpha phosphoinositide 3-kinase in severe insulin resistance: a novel variant with impaired insulin-stimulated lipid kinase activity. Diabetologia 43(3):321–331
Barroso I, Luan J, Middelberg RP, Harding AH, Franks PW, Jakes RW, Clayton D, Schafer AJ, O’Rahilly S, Wareham NJ (2003) Candidate gene association study in type 2 diabetes indicates a role for genes involved in beta-cell function as well as insulin action. PLoS Biol 1(1):E20. doi:10.1371/journal.pbio.0000020
Jamshidi Y, Snieder H, Wang X, Pavitt MJ, Spector TD, Carter ND, O’Dell SD (2006) Phosphatidylinositol 3-kinase p85alpha regulatory subunit gene PIK3R1 haplotype is associated with body fat and serum leptin in a female twin population. Diabetologia 49(11):2659–2667. doi:10.1007/s00125-006-0388-z
Reiter V, Matschkal DM, Wagner M, Globisch D, Kneuttinger AC, Muller M, Carell T (2012) The CDK5 repressor CDK5RAP1 is a methylthiotransferase acting on nuclear and mitochondrial RNA. Nucleic Acids Res 40(13):6235–6240. doi:10.1093/nar/gks240
Liang Q, Li L, Zhang J, Lei Y, Wang L, Liu DX, Feng J, Hou P, Yao R, Zhang Y, Huang B, Lu J (2013) CDK5 is essential for TGF-beta1-induced epithelial-mesenchymal transition and breast cancer progression. Sci Rep 3:2932. doi:10.1038/srep02932
Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, Wellcome Trust Case Control C, McCarthy MI, Hattersley AT (2007) Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316 (5829):1336–1341. doi:10.1126/science.1142364
Pascoe L, Tura A, Patel SK, Ibrahim IM, Ferrannini E, Zeggini E, Weedon MN, Mari A, Hattersley AT, McCarthy MI, Frayling TM, Walker M, Consortium R, Consortium UKTDG (2007) Common variants of the novel type 2 diabetes genes CDKAL1 and HHEX/IDE are associated with decreased pancreatic beta-cell function. Diabetes 56(12):3101–3104. doi:10.2337/db07-0634
Chang CL, Cai JJ, Huang SY, Cheng PJ, Chueh HY, Hsu SY (2014) Adaptive human CDKAL1 variants underlie hormonal response variations at the enteroinsular axis. PLoS ONE 9(9):e105410. doi:10.1371/journal.pone.0105410
Fuentes-Mattei E, Velazquez-Torres G, Phan L, Zhang F, Chou PC, Shin JH, Choi HH, Chen JS, Zhao R, Chen J, Gully C, Carlock C, Qi Y, Zhang Y, Wu Y, Esteva FJ, Luo Y, McKeehan WL, Ensor J, Hortobagyi GN, Pusztai L, Fraser Symmans W, Lee MH, Yeung SC (2014) Effects of obesity on transcriptomic changes and cancer hallmarks in estrogen receptor-positive breast cancer. J Natl Cancer Inst. doi:10.1093/jnci/dju158
Gong Z, Quan L, Yao S, Zirpoli G, Bandera EV, Roberts M, Coignet JG, Cabasag C, Sucheston L, Hwang H, Ciupak G, Davis W, Pawlish K, Jandorf L, Bovbjerg DH, Ambrosone CB, Hong CC (2013) Innate immunity pathways and breast cancer Risk in African American and European-American women in the Women’s Circle of Health Study (WCHS). PLoS ONE 8(8):e72619. doi:10.1371/journal.pone.0072619
Yao S, Graham K, Shen J, Campbell LE, Singh P, Zirpoli G, Roberts M, Ciupak G, Davis W, Hwang H, Khoury T, Bovbjerg DH, Jandorf L, Pawlish KS, Bandera EV, Liu S, Ambrosone CB, Zhao H (2013) Genetic variants in microRNAs and breast cancer risk in African American and European American women. Breast Cancer Res Treat 141(3):447–459. doi:10.1007/s10549-013-2698-4
Shifman S, Kuypers J, Kokoris M, Yakir B, Darvasi A (2003) Linkage disequilibrium patterns of the human genome across populations. Hum Mol Genet 12(7):771–776
Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, Harris TB, Everhart JE, Schenker N (2009) Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr 89(2):500–508. doi:10.3945/ajcn.2008.26847
Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL Jr, Ravussin E, Ryan DH, Smith SR, Bouchard C (2010) Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr 91(1):7–15. doi:10.3945/ajcn.2009.28136
Donohoe CL, Doyle SL, Reynolds JV (2011) Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr 3:12. doi:10.1186/1758-5996-3-12
Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Andrada P, Rotellar F, Valenti V, Moncada R, Marti P, Silva C, Salvador J, Fruhbeck G (2015) Expression of S6K1 in human visceral adipose tissue is upregulated in obesity and related to insulin resistance and inflammation. Acta Diabetol 52(2):257–266. doi:10.1007/s00592-014-0632-9
Acknowledgments
This work was supported in part by grants from the US National Institutes of Health (P01 CA151135, R01 CA100598, K22 CA138563, and P30 CA072720), US Army Medical Research and Material Command (DAMD-17-01-1-0334), the Breast Cancer Research Foundation, and a gift from the Philip L. Hubbell family. The study used shared resources supported by Roswell Park Cancer Institute’s Cancer Center Support Grant from the National Cancer Institute (P30CA016056). The New Jersey State Cancer Registry is supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention under cooperative agreement 1US58DP003931-01 awarded to the New Jersey Department of Health. The collection of New Jersey cancer incidence data is also supported by the Surveillance, Epidemiology, and End Results program of the National Cancer Institute under contract N01-PC-2010-0027 and the State of New Jersey. The authors thank Dr. Dana Bovbjerg, Ms. Lina Jandorf, and Ms. Edie Prescod for their contribution to the Women’s Circle of Health Study. We also thank our research personnel at the Cancer Institute of New Jersey (now Rutgers Cancer Institute of New Jersey), Roswell Park Cancer Institute, Mount Sinai School of Medicine (now Icahn School of Medicine at Mount Sinai), UMDNJ School of Public Health (now Rutgers School of Public Health), and the New Jersey State Cancer Registry, as well as our African-American breast cancer advocates and community partners, and all the women who generously donated their time to participate in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheng, TY.D., Shankar, J., Zirpoli, G. et al. Genetic variants in the mTOR pathway and interaction with body size and weight gain on breast cancer risk in African-American and European American women. Cancer Causes Control 27, 965–976 (2016). https://doi.org/10.1007/s10552-016-0774-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-016-0774-x