Abstract
Purpose
The findings from epidemiologic studies of dyslipidemia and colorectal cancer risk have been conflicting. We performed a dose–response meta-analysis of published prospective studies to assess the aforementioned association.
Methods
Relevant studies that reported the association between the components of dyslipidemia (serum triglyceride, total cholesterol, and high-/low-density lipoprotein cholesterol) and colorectal cancer risk were identified by searching PubMed until the end of May 2014. We pooled the relative risks (RRs) from individual studies using a random- and fixed-effects models and performed dose–response, heterogeneity, and publication bias analyses.
Results
Seventeen prospective studies, including 1,987,753 individuals with 10,876 colorectal cancer events, were included in the meta-analysis. The overall pooled RR for high versus low concentrations for triglyceride (n = 9 studies) was 1.18 (95 % CI 1.04–1.34; I 2 = 47.8 %), for total cholesterol (n = 10 studies) was 1.11 (95 % CI 1.01–1.21; I 2 = 46.7 %), for high-density lipoprotein cholesterol (n = 6 studies) was 0.84 (95 % CI 0.69–1.02; I 2 = 42.5 %), and for low-density lipoprotein cholesterol (n = 3 studies) was 1.04 (95 % CI 0.60–1.81; I 2 = 82.7 %). In the dose–response analysis, the overall pooled RR was 1.01 (95 % CI 1.00–1.03; I 2 = 0 %) per 50 mg/dL of triglyceride and 1.01 (95 % CI 0.97–1.05; I 2 = 64.3 %) per 100 mg/dL of total cholesterol.
Conclusions
This meta-analysis of prospective studies suggests that dyslipidemia, especially high levels of serum triglyceride and total cholesterol, is associated with an increased risk of colorectal cancer, whereas high-density lipoprotein cholesterol might associate with a decreased risk of colorectal cancer. Further studies are warranted to determine whether altering the concentrations of these metabolic variables may reduce colorectal cancer risk.



Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray, F. (2013) GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC cancer base no. 11 [Internet]. International agency for research on cancer, Lyon, France. http://globocan.iarc.fr. Accessed 14 July 2014
World Cancer Resarch Fund/American Institue for Cancer Research (2007) Food, nutrition, physical activity and the prevention of cancer: a global perspective. AICR, Washington
Bardou M, Barkun AN, Martel M (2013) Obes colorectal Cancer. Gut 62:933–947
Giovannucci E (2007) Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 86:s836–s842
Esteve E, Ricart W, Fernandez-Real JM (2005) Dyslipidemia and inflammation: an evolutionary conserved mechanism. Clin Nutr 24:16–31
Kontush A, de Faria EC, Chantepie S, Chapman MJ (2005) A normotriglyceridemic, low HDL–cholesterol phenotype is characterised by elevated oxidative stress and HDL particles with attenuated antioxidative activity. Atherosclerosis 182:277–285
Vekic J, Kotur-Stevuljevic J, Jelic-Ivanovic Z et al (2007) Association of oxidative stress and PON1 with LDL and HDL particle size in middle-aged subjects. Eur J Clin Invest 37:715–723
Avramoglu RK, Basciano H, Adeli K (2006) Lipid and lipoprotein dysregulation in insulin resistant states. Clin Chim Acta 368:1–19
Ghandehari H, Kamal-Bahl S, Wong ND (2008) Prevalence and extent of dyslipidemia and recommended lipid levels in US adults with and without cardiovascular comorbidities: the National Health and Nutrition Examination Survey 2003–2004. Am Heart J 156:112–119
Rosamond W, Flegal K, Furie K et al (2008) Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117:e25–e146
Ford ES, Mokdad AH, Giles WH, Mensah GA (2003) Serum total cholesterol concentrations and awareness, treatment, and control of hypercholesterolemia among US adults: findings from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 107:2185–2189
Borena W, Stocks T, Jonsson H et al (2011) Serum triglycerides and cancer risk in the metabolic syndrome and cancer (Me-Can) collaborative study. Cancer Causes Control 22:291–299
Inoue M, Noda M, Kurahashi N et al (2009) Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev 18:240–247
Tulinius H, Sigfusson N, Sigvaldason H et al (1997) Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev 6:863–873
Agnoli C, Grioni S, Sieri S et al (2014) Colorectal cancer risk and dyslipidemia: a case–cohort study nested in an Italian multicentre cohort. Cancer Epidemiol 38:144–151
Kitahara CM, Berrington DGA, Freedman ND et al (2011) Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol 29:1592–1598
Tornberg SA, Holm LE, Carstensen JM, Eklund GA (1986) Risks of cancer of the colon and rectum in relation to serum cholesterol and beta-lipoprotein. N Engl J Med 315:1629–1633
Strohmaier S, Edlinger M, Manjer J et al (2013) Total serum cholesterol and cancer incidence in the Metabolic syndrome and Cancer Project (Me-Can). PLoS One 8:e54242
van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M et al (2011) Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European prospective investigation into cancer and nutrition. Gut 60:1094–1102
Ahn J, Lim U, Weinstein SJ et al (2009) Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol Biomarkers Prev 18:2814–2821
Iso H, Ikeda A, Inoue M et al (2009) Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int J Cancer 125:2679–2686
Ahmed RL, Schmitz KH, Anderson KE et al (2006) The metabolic syndrome and risk of incident colorectal cancer. Cancer 107:28–36
Bowers K, Albanes D, Limburg P et al (2006) A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am J Epidemiol 164:652–664
Tsushima M, Nomura AM, Lee J, Stemmermann GN (2005) Prospective study of the association of serum triglyceride and glucose with colorectal cancer. Dig Dis Sci 50:499–505
Saydah SH, Platz EA, Rifai N et al (2003) Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 12:412–418
Schoen RE, Tangen CM, Kuller LH et al (1999) Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst 91:1147–1154
Chyou PH, Nomura AM, Stemmermann GN (1996) A prospective study of colon and rectal cancer among Hawaii Japanese men. Ann Epidemiol 6:276–282
Esposito K, Chiodini P, Capuano A et al (2013) Colorectal cancer association with metabolic syndrome and its components: a systematic review with meta-analysis. Endocrine 44:634–647
Law MR, Thompson SG (1991) Low serum cholesterol and the risk of cancer: an analysis of the published prospective studies. Cancer Causes Control 2:253–261
Giovannucci E (2007) Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 86:s836–s842
Kritchevsky SB, Kritchevsky D (1992) Serum cholesterol and cancer risk: an epidemiologic perspective. Annu Rev Nutr 12:391–416
Sidney S, Farquhar JW (1983) Cholesterol, cancer, and public health policy. Am J Med 75:494–508
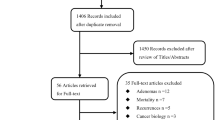
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 339:b2535
Schatzkin A, Hoover RN, Taylor PR et al (1988) Site-specific analysis of total serum cholesterol and incident cancer in the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Cancer Res 48:452–458
Wells GA, O’Connell D, Peterson J, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 15 June 2014
Wu QJ, Yang Y, Vogtmann E et al (2013) Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Ann Oncol 24:1079–1087
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Hamling J, Lee P, Weitkunat R, Ambuhl M (2008) Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 27:954–970
Danesh J, Collins R, Appleby P, Peto R (1998) Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 279:1477–1482
Chene G, Thompson SG (1996) Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol 144:610–621
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135:1301–1309
Orsini N, Li R, Wolk A et al (2012) Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175:66–73
Royston P (2000) A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med 19:1831–1847
Bagnardi V, Zambon A, Quatto P, Corrao G (2004) Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol 159:1077–1086
Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Esposito K, Chiodini P, Capuano A et al (2013) Metabolic syndrome and postmenopausal breast cancer: systematic review and meta-analysis. Menopause 20:1301–1309
Esposito K, Chiodini P, Capuano A et al (2013) Effect of metabolic syndrome and its components on prostate cancer risk: meta-analysis. J Endocrinol Invest 36:132–139
Wei EK, Giovannucci E, Wu K et al (2004) Comparison of risk factors for colon and rectal cancer. Int J Cancer 108:433–442
Sugai T, Habano W, Jiao YF et al (2006) Analysis of molecular alterations in left- and right-sided colorectal carcinomas reveals distinct pathways of carcinogenesis: proposal for new molecular profile of colorectal carcinomas. J Mol Diagn 8:193–201
van Stiphout WA, Hofman A, de Bruijn AM (1987) Serum lipids in young women before, during, and after pregnancy. Am J Epidemiol 126:922–928
Munzer T, Harman SM, Sorkin JD, Blackman MR (2009) Growth hormone and sex steroid effects on serum glucose, insulin, and lipid concentrations in healthy older women and men. J Clin Endocrinol Metab 94:3833–3841
Kumagai S, Kai Y, Sasaki H (2001) Relationship between insulin resistance, sex hormones and sex hormone-binding globulin in the serum lipid and lipoprotein profiles of Japanese postmenopausal women. J Atheroscler Thromb 8:14–20
McKeown-Eyssen G (1994) Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol Biomarkers Prev 3:687–695
Esteve E, Ricart W, Fernandez-Real JM (2005) Dyslipidemia and inflammation: an evolutionary conserved mechanism. Clin Nutr 24:16–31
Erdman SE, Poutahidis T (2010) Roles for inflammation and regulatory T cells in colon cancer. Toxicol Pathol 38:76–87
Kim S, Keku TO, Martin C et al (2008) Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res 68:323–328
Wu H, Jiang H, Lu D et al (2009) Effect of simvastatin on glioma cell proliferation, migration, and apoptosis. Neurosurgery 65:1087
van Exel E, Gussekloo J, de Craen AJ et al (2002) Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes : the Leiden 85-Plus Study. Diabetes 51:1088–1092
Kontush A, de Faria EC, Chantepie S, Chapman MJ (2005) A normotriglyceridemic, low HDL–cholesterol phenotype is characterised by elevated oxidative stress and HDL particles with attenuated antioxidative activity. Atherosclerosis 182:277–285
Vekic J, Kotur-Stevuljevic J, Jelic-Ivanovic Z et al (2007) Association of oxidative stress and PON1 with LDL and HDL particle size in middle-aged subjects. Eur J Clin Invest 37:715–723
Clarke R, Shipley M, Lewington S et al (1999) Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 150:341–353
Al-Delaimy WK, Jansen EH, Peeters PH et al (2006) Reliability of biomarkers of iron status, blood lipids, oxidative stress, vitamin D, C-reactive protein and fructosamine in two Dutch cohorts. Biomarkers 11:370–382
Bonovas S, Filioussi K, Flordellis CS, Sitaras NM (2007) Statins and the risk of colorectal cancer: a meta-analysis of 18 studies involving more than 1.5 million patients. J Clin Oncol 25:3462–3468
Cole BF, Logan RF, Halabi S et al (2009) Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst 101:256–266
Acknowledgments
None of the grant supports this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10552_2014_507_MOESM1_ESM.tif
Supplementary material 1 Supplementary Figure S1. Forest plots (random effect model) of meta-analysis on the relationship between high-density lipoprotein cholesterol concentrations and colorectal cancer risk. Squares indicate study-specific relative risks (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95 % CIs; and diamond indicates the summary relative risk estimate with its 95 % CI. CC: colon cancer; CI: confidence interval; CRC: colorectal cancer; F: female; M: male; RC: rectal cancer; and RR: relative risk. (TIFF 2,577 kb)
10552_2014_507_MOESM2_ESM.tif
Supplementary material 2 Supplementary Figure S2. Forest plots (random effect model) of meta-analysis on the relationship between low-density lipoprotein cholesterol concentrations and colorectal cancer risk. Squares indicate study-specific relative risks (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95 % CIs; and diamond indicates the summary relative risk estimate with its 95 % CI. CC: colon cancer; CI: confidence interval; CRC: colorectal cancer; F: female; M: male; RC: rectal cancer; and RR: relative risk. (TIFF 1,823 kb)
Rights and permissions
About this article
Cite this article
Yao, X., Tian, Z. Dyslipidemia and colorectal cancer risk: a meta-analysis of prospective studies. Cancer Causes Control 26, 257–268 (2015). https://doi.org/10.1007/s10552-014-0507-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-014-0507-y