Abstract
Purpose
To quantify the extent to which a clinically significant prostate cancer mortality reduction due to screening could have been masked by control arm screening (contamination) in the Prostate, Lung, Colorectal, and Ovarian (PLCO) trial.
Methods
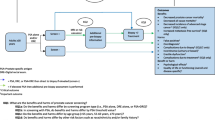
We used three independently developed models of prostate cancer natural history to conduct a virtual PLCO trial. Simulated participants underwent pre-trial screening based on population patterns. The intervention arm followed observed compliance during the trial then resumed population screening. A contaminated control arm followed observed contamination during the trial then resumed population screening, while an uncontaminated control arm discontinued screening upon entry. We assumed a clinically significant screening benefit, applied population treatments and survival patterns, and calculated mortality rate ratios relative to the contaminated and uncontaminated control arms.
Results
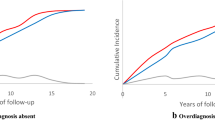
The virtual trial reproduced observed incidence, including stage and grade distributions, and control arm mortality after 10 years of complete follow-up. Under the assumed screening benefit, the three models found that contamination increased the mortality rate ratio from 0.68–0.77 to 0.86–0.91, increased the chance of excess mortality in the intervention arm from 0–4 % to 15–28 %, and decreased the power of the trial to detect a mortality difference from 40–70 % to 9–25 %.
Conclusions
Our computer simulation models indicate that contamination substantially limited the ability of the PLCO to identify a clinically significant screening benefit. While the trial shows annual screening does not reduce mortality relative to population screening, contamination prevents concluding whether screening reduces mortality relative to no screening.




Similar content being viewed by others

References
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Maattanen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A (2009) Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 360(13):1320–1328
Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, Fouad MN, Isaacs C, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O’Brien B, Ragard LR, Clapp JD, Rathmell JM, Riley TL, Hsing AW, Izmirlian G, Pinsky PF, Kramer BS, Miller AB, Gohagan JK, Prorok PC (2012) Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian cancer screening trial: mortality results after 13 years of follow-up. J Natl Cancer Inst 104:1–8. doi:10.1093/jnci/djr500
Pinsky PF, Black A, Kramer BS, Miller A, Prorok PC, Berg C (2010) Assessing contamination and compliance in the prostate component of the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial. Clin Trials 7(4):303–311. doi:10.1177/1740774510374091
Berg CD (2011) The Prostate, Lung, Colorectal and Ovarian cancer screening trial: the prostate cancer screening results in context. Acta Oncol 50(Suppl 1):12–17. doi:10.3109/0284186X.2010.531283
Chou R, Croswell JM, Dana T, Bougatsos C, Blazina I, Fu R, Gleitsmann K, Koenig HC, Lam C, Maltz A, Rugge JB, Lin K (2011) Screening for prostate cancer: a review of the evidence for the U.S. preventive services task force. Ann Intern Med. doi:10.1059/0003-4819-155-11-201112060-00375
Cuzick J, Edwards R, Segnan N (1997) Adjusting for non-compliance and contamination in randomized clinical trials. Stat Med 16(9):1017–1029. doi:10.1002/(SICI)1097-0258(19970515)16:9<1017:AID-SIM508>3.0.CO;2-V
Roobol MJ, Kerkhof M, Schroder FH, Cuzick J, Sasieni P, Hakama M, Stenman UH, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis L, Recker F, Berenguer A, Ruutu M, Kujala P, Bangma CH, Aus G, Tammela TL, Villers A, Rebillard X, Moss SM, de Koning HJ, Hugosson J, Auvinen A (2009) Prostate cancer mortality reduction by prostate-specific antigen-based screening adjusted for nonattendance and contamination in the European Randomised Study of Screening for Prostate Cancer (ERSPC). Eur Urol 56(4):584–591. doi:10.1016/j.eururo.2009.07.018
Draisma G, Boer R, Otto SJ, van der Cruijsen IW, Damhuis RA, Schröder FH, de Koning HJ (2003) Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst 95(12):868–878
Tsodikov A, Szabo A, Wegelin J (2006) A population model of prostate cancer incidence. Stat in Med 25(16):2846–2866
Gulati R, Inoue L, Katcher J, Hazelton W, Etzioni R (2010) Calibrating disease progression models using population data: a critical precursor to policy development in cancer control. Biostatistics 11(4):707–719. doi:10.1093/biostatistics/kxq036
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality—All COD, Public-Use With State, Total U.S. (1969–2005), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008. Underlying mortality data provided by NCHS (www.cdc.gov/nchs)
Mariotto A, Etzioni R, Krapcho M, Feuer EJ (2007) Reconstructing prostate-specific antigen (PSA) testing patterns among black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer 109(9):1877–1886
Goodman PJ, Thompson IM Jr, Tangen CM, Crowley JJ, Ford LG, Coltman CA Jr (2006) The Prostate Cancer Prevention Trial: design, biases and interpretation of study results. J Urol 175(6):2234–2242. doi:10.1016/S0022-5347(06)00284-9
Pinsky PF, Crawford ED, Kramer BS, Andriole GL, Gelmann EP, Grubb R, Greenlee R, Gohagan JK (2007) Repeat prostate biopsy in the Prostate, Lung, Colorectal and Ovarian cancer screening trial. BJU Int 99(4):775–779. doi:10.1111/j.1464-410X.2007.06708.x
Grubb RL 3rd, Pinsky PF, Greenlee RT, Izmirlian G, Miller AB, Hickey TP, Riley TL, Mabie JE, Levin DL, Chia D, Kramer BS, Reding DJ, Church TR, Yokochi LA, Kvale PA, Weissfeld JL, Urban DA, Buys SS, Gelmann EP, Ragard LR, Crawford ED, Prorok PC, Gohagan JK, Berg CD, Andriole GL (2008) Prostate cancer screening in the Prostate, Lung, Colorectal and Ovarian cancer screening trial: update on findings from the initial four rounds of screening in a randomized trial. BJU Int 102(11):1524–1530. doi:10.1111/j.1464-410X.2008.08214.x
Eichler K, Hempel S, Wilby J, Myers L, Bachmann LM, Kleijnen J (2006) Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol 175(5):1605–1612
Wever EM, Draisma G, Heijnsdijk EA, Roobol MJ, Boer R, Otto SJ, De Koning HJ (2010) Prostate-specific antigen screening in the United States vs in the European Randomized Study of Screening for Prostate Cancer-Rotterdam. J Natl Cancer Inst 102(5):352–355
Chefo S, Tsodikov A (2009) Stage-specific cancer incidence: an artificially mixed multinomial logit model. Stat Med 28(15):2054–2076
Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O’Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD (2009) Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 360(13):1310–1319
Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, Fogel R, Gelmann EP, Gilbert F, Hasson MA, Hayes RB, Johnson CC, Mandel JS, Oberman A, O’Brien B, Oken MM, Rafla S, Reding D, Rutt W, Weissfeld JL, Yokochi L, Gohagan JK (2000) Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Control Clin Trials 21(6 Suppl):273S–309S
Babaian RJ, Toi A, Kamoi K, Troncoso P, Sweet J, Evans R, Johnston DA, Chen M (2000) A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol 163(1):152–157
Presti JCJ, Chang JJ, Bhargava V, Shinohara K (2000) The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol 163(1):163–166 (discussion 166–167)
Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, Nordling S, Haggman M, Andersson SO, Bratell S, Spangberg A, Palmgren J, Steineck G, Adami HO, Johansson JE (2011) Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 364(18):1708–1717. doi:10.1056/NEJMoa1011967
Wever EM, Draisma G, Heijnsdijk EA, de Koning HJ (2011) How does early detection by screening affect disease progression? Modeling estimated benefits in prostate cancer screening. Med Decis Making. doi:10.1177/0272989X10396717
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Pinsky PF, Miller A, Kramer BS, Church T, Reding D, Prorok P, Gelmann E, Schoen RE, Buys S, Hayes RB, Berg CD (2007) Evidence of a healthy volunteer effect in the Prostate, Lung, Colorectal, and Ovarian cancer screening trial. Am J Epidemiol 165(8):874–881. doi:10.1093/aje/kwk075
Cetin K, Beebe-Dimmer JL, Fryzek JP, Markus R, Carducci MA (2010) Recent time trends in the epidemiology of stage IV prostate cancer in the United States: analysis of data from the surveillance, epidemiology, and end results program. Urology 75(6):1396–1404. doi:10.1016/j.urology.2009.07.1360
Schröder FH, Roobol MJ (2010) ERSPC and PLCO prostate cancer screening studies: what are the differences? Eur Urol 58(1):46–52. doi:10.1016/j.eururo.2010.03.033
Studer UE, Collette L (2010) What can be concluded from the ERSPC and PLCO trial data? Urol Oncol 28(6):668–669. doi:10.1016/j.urolonc.2010.03.011
Croswell JM, Kramer BS, Crawford ED (2011) Screening for prostate cancer with PSA testing: current status and future directions. Oncology 25(6):452–460 (463)
Acknowledgments
We are grateful to Dr. Paul F. Pinsky for careful reading and helpful comments on an earlier draft. Any remaining errors are our own. This research was supported by U01CA157224-01 from the National Cancer Institute and U01CA157224 from the National Cancer Institute and the Centers for Disease Control. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, or the Centers for Disease Control.
Conflict of interest
HJK received partial funding from Beckman Coulter to evaluate cost-effectiveness of the prostate health index. All other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gulati, R., Tsodikov, A., Wever, E.M. et al. The impact of PLCO control arm contamination on perceived PSA screening efficacy. Cancer Causes Control 23, 827–835 (2012). https://doi.org/10.1007/s10552-012-9951-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-012-9951-8