Abstract
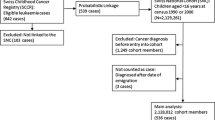
It has been proposed that type 1 diabetes (T1D) and leukemia in children may cluster in space and time due to common spatially mediated etiologies. We investigated this hypothesis and clustering of both diseases separately in Danish children aged 0–14 years, using 1,168 leukemia cases diagnosed in the period 1980–2006, 2,443 T1D cases diagnosed 1996–2006, and population-based controls matched on age, gender, and time of diagnosis. Residential histories from birth to diagnosis were collected. For leukemia in ages 0–14 years, we found no evidence of clustering; we did find spatial clustering at time of diagnosis for children aged 2–6 years with acute lymphoblastic leukemia (ALL) (observed/expected [95% confidence interval]: 1.35 [1.15–1.54]). T1D cases showed clustering at birth for ages 0–14 years; for ages 0–4 years at diagnosis, and when the residential history was accounted for. T1D cases clustered near leukemia cases particularly in the age group 2–6 years at diagnosis. Leukemia and T1D in this age group thus may share etiological factors mediated by geographic location. This suggests common environmental risk factors, with exposure to infections as first possible candidate, geographically localized exposure to agents that compromise development and/or response of the immune system being a second, and chance being a third.


Similar content being viewed by others
References
Feltbower RG, Manda SOM, Gilthorpe MS, Greaves MF, Parslow RC, Kinsey SE, Bodansky HJ, McKinney PA (2005) Detecting small-area similarities in the epidemiology of childhood acute lymphoblastic leukemia and diabetes mellitus, type 1: a Bayesian approach. Am J Epidemiol 161(12):1168–1180. doi:10.1093/aje/kwi146
Manda SOM, Feltbower RG, Gilthorpe MS (2009) Investigating spatio-temporal similarities in the epidemiology of childhood leukaemia and diabetes. Eur J Epidemiol 24(12):743–752. doi:10.1007/s10654-009-9391-2
Svendsen AL, Feychting M, Klæboe L, Langmark F, Schüz J (2007) Time trends in the incidence of acute lymphoblastic leukemia among children 1976–2002: a population-based Nordic study. J Pediatr 151(5):548–550. doi:10.1016/j.jpeds.2007.07.006
Buffler PA, Kwan ML, Reynolds P, Urayama KY (2005) Environmental and genetic risk factors for childhood leukemia: appraising the evidence. Cancer Invest 23(1):60–75. doi:10.1081/CNV-200046402
Svensson J, Carstensen B, Mølbak A, Christau B, Mortensen HB, Nerup J, Borch-Johnsen K (2002) Increased risk of childhood type 1 diabetes in children born after 1985. Diabetes Care 25(12):2197–2201. doi:10.2337/diacare.25.12.2197
Greaves M (2002) Science, medicine, and the future: childhood leukaemia. BMJ 324(7332):283–287. doi:10.1136/bmj.324.7332.283
EURODIAB Study Group (2000) Infections and vaccinations as risk factors for childhood type I (insulin-dependent) diabetes mellitus: a multicentre casecontrol investigation. EURODIAB Substudy 2 Study Group. Diabetologia 43(1):47–53. doi:10.1007/s001250050006
Guarner F, Bourdet-Sicard R, Brandtzaeg P, Gill HS, McGuirk P, van Eden W, Versalovic J, Weinstock JV, Rook GA (2006) Mechanisms of disease: the hygiene hypothesis revisited. Nat Clin Pract Gastroenterol Hepatol 3(5):275–284. doi:10.1038/ncpgasthep0471
Kinlen LJ (1988) Evidence for an infective cause of childhood leukaemia: comparison of a Scottish new town with nuclear reprocessing sites in Britain. Lancet 2(8624):1323–1327. doi:10.1016/S0140-6736(88)90867-7
Steliarova-Foucher E, Stiller C, Kaatsch P, Berrino F, Coebergh J-W, Lacour B, Perkin M (2004) Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970 s (the ACCIS project): an epidemiological study. Lancet 364(9451):2097–2105. doi:10.1016/S0140-6736(04)17550-8
Green A, Patterson CC, EURODIAB TIGER Study Group (2001) Europe and Diabetes Trends in the incidence of childhood-onset diabetes in Europe 1989–1998. Diabetologia 44(suppl 3):B3–B8. doi:10.1007/PL00002950
Kulldorff M (2003) Statistical Methods for Spatial Epidemiology: Tests for Randomness. In: Gatrell AC, Löytönen M (eds) GIS and Health. Taylor & Francis Ltd, London, pp 49–62
The Danish National Board of Health, Cancer incidence in Denmark (2001)
Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P (2005) International classification of childhood cancer, third edition. Cancer 103(7):1457–1467. doi:10.1002/cncr.20910
Haines L, Wan KC, Lynn R, Barrett TG, Shield JP (2007) Rising incidence of type 2 diabetes in children in the UK. Diabetes Care 30(5):1097–1101. doi:10.2337/dc06-1813
Diggle PJ, Chetwynd AG (1991) Second-order analysis of spatial clustering for inhomogeneous populations. Biometrics 47(3):1155–1163. doi:10.2307/2532668
Cuzick J, Edwards R (1990) Spatial Clustering for Inhomogeneous Populations. J R Stat Soc (Ser B) 52(1):73–104
Jacquez GM, Kaufmann A, Meliker J, Goovaerts P, AvRuskin G, Nriagu J (2005) Global, local and focused geographic clustering for case-control data with residential histories. Environ Health 4(1):4. doi:10.1186/1476-069X-4-4
R Development Core Team (2010) R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org
Jacquez GM (2004) Current practices in the spatial analysis of cancer: flies in the ointment. Int J Health Geogr 3:22. doi:10.1186/1476-072X-3-22
Committee on Medical Aspects of Radiation in the Environment (COMARE) (2006) Eleventh Report. The distribution of childhood leukaemia and other childhood cancer in Great Britain 1969-1993. Health Protection Agency, July 2006
Alexander FE, Boyle P, Carli PM, Coebergh JW, Draper GJ, Ekbom A, Levi F, McKinney PA, McWhirter W, Michaelis J, Peris-Bonet R, Petridou E, Pompe-Kirn V, Plìsko I, Pukkala E, Rahu M, Storm H, Terracini B, Vatten L, Wray N (1998) Spatial clustering of childhood leukaemia: summary results from the EUROCLUS project. Br J Cancer 77(5):818–824. doi:10.1038/bjc.1998.133
Muir KR, Parkes SE, Mann JR, Stevens MC, Cameron AH, Raafat F, Darbyshire PJ, Ingram DR, Davis A, Gascoigne D (1990) Clustering’—real or apparent? Probability maps of childhood cancer in the West Midlands Health Authority Region. Int J Epidemiol 19(4):853–859. doi:10.1093/ije/19.4.853
Hjalmars U, Kulldorff M, Gustafsson G, Nagarwalla N (1996) Childhood leukaemia in Sweden: using GIS and a spatial scan statistic for cluster detection. Stat Med 15(7–9):707–715. doi:10.1002/(SICI)1097-0258(19960415)15:7/9<707:AID-SIM242>3.0.CO;2-4
Mosavi-Jarrahi A, Moini M, Mohagheghi MA, Alebouyeh M, Yazdizadeh B, Shahabian A, Nahvijo A, Alizadeh R (2007) Clustering of childhood cancer in the inner city of Tehran metropolitan area: a GIS-based analysis. Int J Hyg Environ Health 210(2):113–119. doi:10.1016/j.ijheh.2006.08.005
Westermeier T, Michaelis J (1995) Applicability of the Poisson distribution to model the data of the German Children’s Cancer Registry. Radiat Environ Biophys 34(1):7–11. doi:10.1007/BF01210539
Schmiedel S, Blettner M, Kaatsch P, Schüz J (2010) Spatial clustering and space-time clusters of leukemia among children in Germany, 1987–2007. Eur J Epidemiol 25(9):627–633. doi:10.1007/s10654-010-9488-7
Alexander FE, Chan LC, Lam TH, Yuen P, Leung NK, Ha SY, Yuen HL, Li CK, Li CK, Lau YL, Greaves MF (1997) Clustering of childhood leukaemia in Hong Kong: association with the childhood peak and common acute lymphoblastic leukaemia and with population mixing. Br J Cancer 75(3):457–463. doi:10.1038/bjc.1997.77
Dockerty JD, Sharples KJ, Borman B (1999) An assessment of spatial clustering of leukaemias and lymphomas among young people in New Zealand. J Epidemiol Community Health 53(3):154–158. doi:10.1136/jech.53.3.154
McNally RJQ, Alexander FE, Birch JM (2002) Space–time clustering analyses of childhood acute lymphoblastic leukaemia by immunophenotype. Br J Cancer 87(5):513–515. doi:10.1038/sj.bjc.6600498
Bellec S, Hémon D, Rudant J, Goubin A, Clavel J (2006) Spatial and space–time clustering of childhood acute leukaemia in France from 1990 to 2000: a nationwide study. Br J Cancer 94(5):763–770. doi:10.1038/sj.bjc.6602980
Knox EG, Gilman EA (1996) Spatial clustering of childhood cancers in Great Britain. J Epidemiol Community Health 50(3):313–319. doi:10.1136/jech.50.3.313
Petridou E, Alexander FE, Trichopoulos D, Revinthi K, Dessypris N, Wray N, Haidas S, Koliouskas D, Kosmidis H, Piperopoulou F, Tzortzatou F (1997) Aggregation of childhood leukemia in geographic areas of Greece. Cancer Causes Control 8(2):239–245. doi:10.1023/A:1018480515690
McNally RJQ, Alexander FE, Vincent TJ, Murphy MF (2009) Spatial clustering of childhood cancer in Great Britain during the period 1969–1993. Int J Cancer 124(4):932–936. doi:10.1002/ijc.23965
Birch JM, Alexander FE, Blair V, Eden OB, Taylor GM, McNally RJQ (2000) Space–time clustering patterns in childhood leukaemia support a role for infection. Br J Cancer 82(9):1571–1576. doi:10.1054/bjoc.1999.1072
Gilman EA, Knox EG (1995) Childhood cancers: space–time distribution in Britain. J Epidemiol Community Health 49(2):158–163. doi:10.1136/jech.49.2.158
Gustafsson B, Carstensen J (2000) Space–time clustering of childhood lymphatic leukaemias and non-Hodgkin’s lymphomas in Sweden. Eur J Epidemiol 16(12):1111–1116
Feltbower RG, McKinney P, Greaves M, Parslow RC, Bodansky H (2004) International parallels in leukaemia and diabetes epidemiology. Arch Dis Child 89(1):54–56
Author information
Authors and Affiliations
Corresponding author
Additional information
Sven Schmiedel is supported by an international PhD stipend from the The Danish Strategic Research Council (Grant number 645-06-0479).
Appendices
Appendix A
Kernel density
Place a kernel at grid point i (i = 1,…,n). Each observed point location j (j = 1,…,n) is then assigned a weight according to the kernel function \( k\left( {d_{ij} } \right) \), which is a function of the distance from grid point i to point location j. The intensity estimate \( \hat{\lambda }_{i} \) at location i is the sum of the individual contributions mode from each observed point j: \( \hat{\lambda }_{i} = \sum\nolimits_{j\left( 1 \right)}^{n} {k\left( {d_{ij} } \right)} \).
There are many possible kernel functions. We used the most common one, the quartic kernel: \( k\left( {d_{ij} } \right) = {\frac{3}{{\pi \tau^{2} }}}\left( {1 - {\frac{{d_{ij}^{2} }}{{\tau^{2} }}}} \right)^{2} ;\quad d_{ij} < \tau \), where τ is the bandwidth of the kernel.
Appendix B
k-function
The k-function is a measure of the second-order characteristics of a point process. It is defined by the mean number of events within a distance h of an arbitrary event, divided by the mean number of events per unit area. The expected value of k(h) in a random pattern is equal to λπh²/λ = πh², where λ is the mean number of events per unit area. An estimate of k(h) is based on the count of number of pairs of points that are found within a distance h of one another, more formally, \( \hat{k}\left( h \right) = {\frac{1}{{\hat{\lambda }^{2} R}}}\sum\nolimits_{i = 1}^{n} {\sum\nolimits_{j \ne i} {I_{ij} \left( h \right)} } \) where R is the area of the study region, n is the number of points observed in R, and I ij (h) is an indicator function equal to one if points i and j are separated by a distance less than h.
Edge effects are incorporated by dividing the I ij (h) term by w ij , defined as the proportion of the circumference of a circle centered on i and passing through j that lies within the study region.
Appendix C
Cuzick–Edwards test
Cuzick–Edwards test is based on the neighboring structure in case–control data. It is the sum of the number of cases among the k-nearest neighbors of all cases, more formally,
\( T_{k} = \sum\nolimits_{i = 1}^{n} {\sum\nolimits_{j = 1}^{n} {w_{ij} \delta_{i} \delta_{j} } } , \) where δ i is equal to one if i is a case, and zero if location i is a control. The term w ij is equal to one if j is a k-nearest neighbor of i and zero otherwise. The choice of k determines the spatial scale at which the test is carried out.
Rights and permissions
About this article
Cite this article
Schmiedel, S., Jacquez, G.M., Blettner, M. et al. Spatial clustering of leukemia and type 1 diabetes in children in Denmark. Cancer Causes Control 22, 849–857 (2011). https://doi.org/10.1007/s10552-011-9755-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-011-9755-2