Abstract
Introduction
We present a large feasibility evaluation of high-risk HPV (HR-HPV) DNA testing and cervical cytology as a primary screening strategy for cervical cancer precursor lesions in Mexican women, as part of a routine cancer control program (CCP).
Methods
A community-based study was carried out in 50,159 women aged 20–70 years who visited the CCP in 12 federal entities located in Northern, Central, and Southern Mexico, including a total of 48 primary health care units of the Instituto Mexicano del Seguro Social (IMSS). Cervical specimens for cytology and HR-HPV tests were collected at baseline. Women with cytological abnormalities (ASCUS or greater) were referred to colposcopy for further evaluation and treatment if necessary. A subset of HR-HPV-positive women without cervical lesions, in Morelos state, were tested again for HR-HPV DNA within a year, and repeat-positive women were referred to colposcopy.
Results
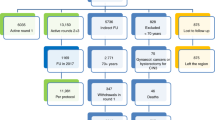
HR-HPV prevalence among all women was 8.6% (95% CI: 8.3–8.9). Prevalence by age group was 12.2% (95% CI: 11.0–13.3) before 30 years of age and decreased to 7.4% (95% CI: 6.7–8.0) between 46 and 50 years of age. A second minor prevalence peak (8.1%; 95% CI: 7.2–9.0) was observed in women more than 55 years of age. Overall prevalence of cytological abnormalities was relatively low (2.2%; 95% CI: 2.0–2.3) with the highest frequency of abnormal cytology (ASCUS or greater) in the 41–45 year age group (2.5%: 95% CI 2.1–2.7). No correlation between cervical abnormalities and HR-HPV prevalence, by region, was observed. A total of 370 (0.7%) women had an abnormal cytology as well as a positive HR-HPV result; 736 (1.5%) had an abnormal cytology and a negative HR-HPV test; 3,941 (7.9%) women had a positive HR-HPV test and a normal cytology; and 45,112 (89.9%) women were negative in both tests. The first two groups were immediately referred to colposcopy, 72.7% of the women from the cytology-positive and HR-HPV-positive group and 58.0% from the cytology-positive and HR-HPV-negative group successfully completing evaluation. Among the 269 cytology-positive and HR-HPV-positive women, 53 (19.7%) CIN2/3+ cases were detected, whereas among the 427 cytology-positive and HR-HPV-negative participants, only 13 (3.0%) CIN2/3+ cases were documented. In Morelos state, a sample of 287 women with a negative cytology smear and a positive HR-HPV test at baseline were re-screened after ~12 months, by means of cytology and HR-HPV testing. Among these women, 106 (36.9%) were again HR-HPV positive and were referred to colposcopy. Of whom, 76 (71.7%) were successfully evaluated; among these women, 9 CIN2/3+ (11.8%) were documented. Sensitivity of cervical cytology for detecting histologically confirmed CIN2/3+ cases was only 40.0% (95% CI 38.5–41.4) compared to 93.3% (95% CI 92.5–94.0) for HPV DNA testing considering the additional cases detected among women with persistent HPV infection. The specificity of cytology was 97.0 vs. 89.2% for the HPV DNA test.
Discussion
Population-based programs using HR-HPV testing can improve cervical cancer prevention and control in Mexican and other populations where cytological screening is inadequate for detecting precursors of cervical cancer.



Similar content being viewed by others
References
Ogedegbe G, Cassells AN, Robinson CM, DuHamel K, Tobin JN, Sox CH, Dietrich AJ (2005) Perceptions of barriers and facilitators of cancer early detection among low-income minority women in community health centers. J Natl Med Assoc 97(2):162–170
Lazcano-Ponce E, Alonso P, Ruiz-Moreno JA, Hernández-Avila M (2003) Recommendations for cervical cancer screening programs in developing countries. The need for equity and technological development. Salud Publica Mex 45(Suppl 3):S449–S462
Lazcano-Ponce EC, Castro R, Allen B, Nájera P, Alonso de Ruíz PA, Hernández-Avila M (1999) Barriers to early detection of cervical-uterine cancer in Mexico. J Womens Health 8(3):399–408
Chu KC, Miller BA, Springfield SA (2007) Measures of racial/ethnic health disparities in cancer mortality rates and the influence of socioeconomic status. J Natl Med Assoc 99(10):1092–1100 (1102–1104)
Raab SS, Jones BA, Souers R, Tworek JA (2008) The effect of continuous monitoring of cytologic-histologic correlation data on cervical cancer screening performance. Arch Pathol Lab Med 132(1):16–22
Tworek JA, Jones BA, Raab S, Clary KM, Walsh MK (2007) The value of monitoring human papillomavirus DNA results for Papanicolaou tests diagnosed as atypical squamous cells of undetermined significance: a College of American Pathologists Q-Probes study of 68 institutions. Arch Pathol Lab Med 131(10):1525–1531
DeMay RM (1996) Cytopathology of false negatives preceding cervical carcinoma. Am J Obstet Gynecol 175(4 Pt 2):1110–1113
Behbakht K, Lynch A, Teal S, Degeest K, Massad S (2004) Social and cultural barriers to Papanicolaou test screening in an urban population. Obstet Gynecol 104(6):1355–1361
Branca M, Ciotti M, Giorgi C, Santini D, Di Bonito L, Costa S, Benedetto A, Bonifacio D, Di Bonito P, Paba P, Accardi L, Syrjänen S, Favalli C, Syrjänen K, on behalf of the HPV-Pathogen ISS Study Group (2008) Predicting high-risk human papillomavirus infection, progression of cervical intraepithelial neoplasia, and prognosis of cervical cancer with a panel of 13 biomarkers tested in multivariate modeling. Int J Gynecol Pathol
Baseman JG, Kulasingam SL, Harris TG, Hughes JP, Kiviat NB, Mao C, Koutsky LA (2008) Evaluation of primary cervical cancer screening with an oncogenic human papillomavirus DNA test and cervical cytologic findings among women who attended family planning clinics in the United States. Am J Obstet Gynecol
Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, Gillio-Tos A, Minucci D, Naldoni C, Rizzolo R, Schincaglia P, Volante R, Zappa M, Zorzi M, Cuzick J, Segnan N, New Technologies for Cervical Cancer Screening Working Group (2008) Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst 100(7):492–501
Naucler P, Ryd W, Törnberg S, Strand A, Wadell G, Elfgren K, Rådberg T, Strander B, Forslund O, Hansson BG, Hagmar B, Johansson B, Rylander E, Dillner J (2009) Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 101(2):88–99
Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, Ratnam S, Coutlée F, Franco EL, Canadian Cervical Cancer Screening Trial Study Group (2007) Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 357(16):1579–1588
Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, Voorhorst FJ, Verheijen RH, van Groningen K, Boon ME, Ruitinga W, van Ballegooijen M, Snijders PJ, Meijer CJ (2007) Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 370(9601):1764–1772
Kotaniemi-Talonen L, Nieminen P, Anttila A, Hakama M (2005) Routine cervical screening with primary HPV testing and cytology triage protocol in a randomised setting. Br J Cancer 93(8):862–867
Kitchener HC, Almonte M, Thomson C, Wheeler P, Sargent A, Stoykova B, Gilham C, Baysson H, Roberts C, Dowie R, Desai M, Mather J, Bailey A, Turner A, Moss S, Peto J (2009) HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 10(7):672–682
Cuzick J, Szarewski A, Mesher D, Cadman L, Austin J, Perryman K, Ho L, Terry G, Sasieni P, Dina R, Soutter WP (2008) Long-term follow-up of cervical abnormalities among women screened by HPV testing and cytology-results from the Hammersmith study. Int J Cancer
Kulasingam SL, Hughes JP, Kiviat NB, Mao C, Weiss NS, Kuypers JM, Koutsky LA (2002) Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 288(14):1749–1757
Leinonen M, Nieminen P, Kotaniemi-Talonen L, Malila N, Tarkkanen J, Laurila P, Anttila A (2009) Age-specific evaluation of primary human papillomavirus screening vs conventional cytology in a randomized setting. J Natl Cancer Inst 101(23):1612–1623
Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D, 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference (2007) 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol 197(4):346–355
Bistoletti P, Sennfält K, Dillner J (2008) Cost-effectiveness of primary cytology and HPV DNA cervical screening. Int J Cancer 122(2):372–376
Goldhaber-Fiebert JD, Stout NK, Salomon JA, Kuntz KM, Goldie SJ (2008) Cost-effectiveness of cervical cancer screening with human papillomavirus DNA testing and HPV-16, 18 vaccination. J Natl Cancer Inst 100(5):308–320
Wheeler CM (2007) Advances in primary and secondary interventions for cervical cancer: human papillomavirus prophylactic vaccines and testing. Nat Clin Pract Oncol 4(4):224–235
Meijer CJ, Berkhof J, Castle PE, Hesselink AT, Franco EL, Ronco G, Arbyn M, Bosch FX, Cuzick J, Dillner J, Heideman DA, Snijders PJ (2008) Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer 124(3):516–520
Dillner J, Rebolj M, Birembaut P, Petry KU, Szarewski A, Munk C, de Sanjose S, Naucler P, Lloveras B, Kjaer S, Cuzick J, van Ballegooijen M, Clavel C, Iftner T, Joint European Cohort Study (2008) Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 337:a1754
Jhala D, Eltoum I (2007) Barriers to adoption of recent technology in cervical screening. Cytojournal 4:16
(2008) Is HPV testing better than a pap test? Not just yet. Mayo Clin Womens Healthsource 12(4):3
Howell LP, Zhou H, Wu W, Davis R (2004) Significance of subclassifying high-grade squamous intraepithelial lesions into moderate dysplasia/CIN II versus severe dysplasia/CIN III/CIS in the Bethesda System terminology. Diagn Cytopathol 30(5):362–366
Lörincz AT (1996) Hybrid Capture method for detection of human papillomavirus DNA in clinical specimens: a tool for clinical management of equivocal Pap smears and for population screening. J Obstet Gynaecol Res 22(6):629–636
Fagan T (1999) Exact 95% confidence intervals for differences in binomial proportions. Comput Biol Med 29:83–87
Ronco G, Cuzick J, Segnan N, Brezzi S, Carozzi F, Folicaldi S, Dalla Palma P, Del Mistro A, Gillio-Tos A, Giubilato P, Naldoni C, Polla E, Iossa A, Zorzi M, Confortini M, Giorgi-Rossi P, NTCC working group (2007) HPV triage for low grade (L-SIL) cytology is appropriate for women over 35 in mass cervical cancer screening using liquid based cytology. Eur J Cancer 43(3):476–480
Sheriff SK, Petry KU, Ikenberg H, Crouse G, Mazonson PD, Santas CC (2007) An economic analysis of human papillomavirus triage for the management of women with atypical and abnormal Pap smear results in Germany. Eur J Health Econ
Reid-Nicholson M, Gatscha RM, Riedel ER, Lin O (2007) Atypical squamous cells, cannot exclude high grade intraepithelial lesion (ASC-H): does HPV matter? Diagn Cytopathol 35(1):1–5
Raab SS, Jones BA, Souers R, Tworek JA (2008) The effect of continuous monitoring of cytologic-histologic correlation data on cervical cancer screening performance. Arch Pathol Lab Med 132(1):16–22
Lazcano-Ponce E, Palacio-Mejia LS, Allen-Leigh B, Yunes-Diaz E, Alonso P, Schiavon R, Hernandez-Avila M (2008) Decreasing cervical cancer mortality in Mexico: effect of Papanicolaou coverage, birthrate, and the importance of diagnostic validity of cytology. Cancer Epidemiol Biomarkers Prev 17(10):2808–2817
Eltoum IA, Roberson J (2007) Impact of HPV testing, HPV vaccine development, and changing screening frequency on national Pap test volume: projections from the National Health Interview Survey (NHIS). Cancer 111(1):34–40
Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahé C, Wright TC, Alliance for Cervical Cancer Prevention Cost Working Group (2005) Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med 353(20):2158–2168
Goldie SJ, Kim JJ, Myers E (2006) Chapter 19: cost-effectiveness of cervical cancer screening. Vaccine 24(Suppl 3):S164–S170
Kimball KJ, Huh WK (2008) Cytology versus high-risk HPV testing for follow-up of HPV-positive women without CIN. J Natl Compr Canc Netw 6(1):96–100
Syrjänen S, Shabalova IP, Petrovichev N, Kozachenko VP, Zakharova T, Pajanidi J, Podistov JI, Chemeris G, Sozaeva LG, Lipova EV, Tsidaeva I, Ivanchenko OG, Pshepurko AA, Zakharenko S, Nerovjna R, Kljukina LB, Erokhina OA, Branovskaja MF, Nikitina M, Grunberga V, Grunberg A, Juschenko A, Tosi P, Cintorino M, Santopietro R, Syrjänen KJ (2002) Human papillomavirus testing and conventional Pap smear cytology as optional screening tools of women at different risks for cervical cancer in the countries of the former Soviet Union. J Low Genit Tract Dis 6(2):97–110
Longatto-Filho A, Erzen M, Branca M, Roteli-Martins C, Naud P, Derchain SF, Hammes L, Sarian LO, Bragança JF, Matos J, Gontijo R, Lima T, Maeda MY, Tatti S, Syrjänen S, Dores G, Lörincz A, Syrjänen K (2006) Human papillomavirus testing as an optional screening tool in low-resource settings of Latin America: experience from the Latin American Screening study. Int J Gynecol Cancer 16(3):955–962
Franco EL, Cuzick J, Hildesheim A, de Sanjosé S (2006) Chapter 20: issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine 24(Suppl 3):S171–S177
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lazcano-Ponce, E., Lörincz, A.T., Salmerón, J. et al. A pilot study of HPV DNA and cytology testing in 50,159 women in the routine Mexican Social Security Program. Cancer Causes Control 21, 1693–1700 (2010). https://doi.org/10.1007/s10552-010-9598-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-010-9598-2