Abstract
Objective
To examine the association between occupational exposure to silica and lung cancer from a systematic review (and meta-analysis) of the epidemiologic literature, with special reference to the methodological quality of observational studies.
Methods
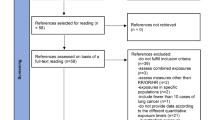
We searched Medline, Toxline, BIOSIS, and Embase (1966–December 2007) for original articles published in any language. Observational studies (cohort and case–control studies) were selected if they reported the result of dose–response analyses relating lung cancer to occupational exposure to silica after appropriate adjustment for smoking.
Results
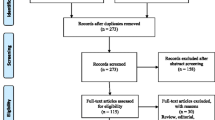
Ten studies (4 cohort studies and 6 case–control studies) met the inclusion criteria of the meta-analysis, nine of which contributing to the main analysis (dose–response analysis, no lag time). We found increasing risk of lung cancer with increasing cumulative exposure to silica, with heterogeneity across studies however. Posthoc analyses identified a set of seven more homogeneous studies. Their meta-analysis resulted in a dose–response curve that was not different from that obtained in the main analysis.
Conclusion
Silica is a lung carcinogen. This increased risk is particularly apparent when the cumulative exposure to silica is well beyond that resulting from exposure to the recommended limit concentration for a prolonged period of time.



Similar content being viewed by others
References
Lacasse Y, Martin S, Simard S, Desmeules M (2005) Meta-analysis of silicosis and lung cancer. Scand J Work Environ Health 31:450–458
Koskela RS, Klockars M, Jarvinen E, Rossi A, Kolari PJ (1990) Cancer mortality of granite workers 1940–1985. IARC Sci Publ (97):43–53
Hughes JM, Weill H (1991) Asbestosis as a precursor of asbestos related lung cancer: results of a prospective mortality study. Br J Ind Med 48:229–233
Hubbard R, Venn A, Lewis S, Britton J (2000) Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med 161:5–8
International Agency for Research on Cancer (IARC) (1997) IARC monographs on the evaluation of carcinogenic risks to humans—silica, some silicates, coal dust and para-aramid fibrils. World Health Organization
Weill H, McDonald JC (1996) Exposure to crystalline silica and risk of lung cancer: the epidemiological evidence. Thorax 51:97–102. doi:10.1136/thx.51.1.97
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012. doi:10.1001/jama.283.15.2008
Gehanno JF, Paris C, Thirion B, Caillard JF (1998) Assessment of bibliographic databases performance in information retrieval for occupational and environmental toxicology. Occup Environ Med 55:562–566. doi:10.1136/oem.55.8.562
Hill AB (1965) The environment and disease: association or causation? Proc R Soc Med 58:295–300
Lagiou P, Adami HO, Trichopoulos D (2005) Causality in cancer epidemiology. Eur J Epidemiol 20:565–574. doi:10.1007/s10654-005-7968-y
Steenland K, Mannetje A, Boffetta P et al (2001) Pooled exposure-response analyses and risk assessment for lung cancer in 10 cohorts of silica-exposed workers: An IARC multicentre study. Cancer Causes Control 12:773–784. doi:10.1023/A:1012214102061
Steenland K, Loomis D, Shy C, Simonsen N (1996) Review of occupational lung carcinogens. Am J Ind Med 29:474–490. doi:10.1002/(SICI)1097-0274(199605)29:5<474::AID-AJIM6>3.0.CO;2-M
Puntoni R, Goldsmith DF, Valerio F et al (1988) A cohort study of workers employed in a refractory brick plant. Tumori 74:27–33
Kramer MS, Feinstein AR (1981) Clinical biostatistics. LIV. The biostatistics of concordance. Clin Pharmacol Ther 29:111–123
Rothman KJ, Greenland S (1998) Modern epidemiology. Lippincott-Raven Publishers, Philadelphia
Il’yasova D, Hertz-Picciotto I, Peters U, Berlin JA, Poole C (2005) Choice of exposure scores for categorical regression in meta-analysis: a case study of a common problem. Cancer Causes Control 16:383–388. doi:10.1007/s10552-004-5025-x
Bagnardi V, Zambon A, Quatto P, Corrao G (2004) Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol 159:1077–1086. doi:10.1093/aje/kwh142
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. doi:10.1016/0197-2456(86)90046-2
Stram DO (1996) Meta-analysis of published data using a linear mixed-effects model. Biometrics 52:536–544. doi:10.2307/2532893
Bonovas S, Filioussi K, Tsavaris N, Sitaras NM (2006) Statins and cancer risk: a literature-based meta-analysis and meta-regression analysis of 35 randomized controlled trials. J Clin Oncol 24:4808–4817. doi:10.1200/JCO.2006.06.3560
Hnizdo E, Murray J, Klempman S (1997) Lung cancer in relation to exposure to silica dust, silicosis and uranium production in South African gold miners. Thorax 52:271–275
Checkoway H, Hughes JM, Weill H, Seixas NS, Demers PA (1999) Crystalline silica exposure, radiological silicosis, and lung cancer mortality in diatomaceous earth industry workers. Thorax 54:56–59
Chen W, Chen J (2002) Nested case-control study of lung cancer in four Chinese tin mines. Occup Environ Med 59:113–118. doi:10.1136/oem.59.2.113
Calvert GM, Rice FL, Boiano JM, Sheehy JW, Sanderson WT (2003) Occupational silica exposure and risk of various diseases: an analysis using death certificates from 27 states of the United States. Occup Environ Med 60:122–129. doi:10.1136/oem.60.2.122
Kauppinen T, Heikkila P, Partanen T et al (2003) Mortality and cancer incidence of workers in Finnish road paving companies. Am J Ind Med 43:49–57. doi:10.1002/ajim.10161
Menvielle G, Luce D, Fevotte J et al (2003) Occupational exposures and lung cancer in New Caledonia. Occup Environ Med 60:584–589. doi:10.1136/oem.60.8.584
Attfield MD, Costello J (2004) Quantitative exposure-response for silica dust and lung cancer in Vermont granite workers. Am J Ind Med 45:129–138. doi:10.1002/ajim.10348
Smailyte G, Kurtinaitis J, Andersen A (2004) Mortality and cancer incidence among Lithuanian cement producing workers. Occup Environ Med 61:529–534. doi:10.1136/oem.2003.009936
L’Abbate N, Di Pierri C, Nuzzaco A, Caputo F, Carino M (2005) Radiological and functional progression in silicosis. Med Lav 96:212–221
Baccarelli A, Khmelnitskii O, Tretiakova M et al (2006) Risk of lung cancer from exposure to dusts and fibers in Leningrad Province, Russia. Am J Ind Med 49:460–467. doi:10.1002/ajim.20316
Chen W, Yang J, Chen J, Bruch J (2006) Exposures to silica mixed dust and cohort mortality study in tin mines: exposure-response analysis and risk assessment of lung cancer. Am J Ind Med 49:67–76. doi:10.1002/ajim.20248
Zeka A, Mannetje A, Zaridze D et al (2006) Lung cancer and occupation in nonsmokers: a multicenter case-control study in Europe. Epidemiology 17:615–623. doi:10.1097/01.ede.0000239582.92495.b5
Yu ITS, Tse LA, Leung CC, Wong TW, Tam CM, Chan ACK (2007) Lung cancer mortality among silicotic workers in Hong Kong—no evidence for a link. Ann Oncol 18:1056–1063. doi:10.1093/annonc/mdm089
Checkoway H, Heyer NJ, Seixas NS et al (1997) Dose-response associations of silica with nonmalignant respiratory disease and lung cancer mortality in the diatomaceous earth industry. Am J Epidemiol 145:680–688
Steenland K, Sanderson W (2001) Lung cancer among industrial sand workers exposed to crystalline silica. Am J Epidemiol 153:695–703. doi:10.1093/aje/153.7.695
Brown TP, Rushton L (2005) Mortality in the UK industrial silica sand industry: 2. A retrospective cohort study. Occup Environ Med 62:446–452. doi:10.1136/oem.2004.017731
Pukkala E, Guo J, Kyyronen P, Lindbohm ML, Sallmen M, Kauppinen T (2005) National job-exposure matrix in analyses of census-based estimates of occupational cancer risk. Scand J Work Environ Health 31:97–107
Ulm K, Waschulzik B, Ehnes H et al (1999) Silica dust and lung cancer in the German stone, quarrying, and ceramics industries: results of a case-control study. Thorax 54:347–351
Bruske-Hohlfeld I, Mohner M, Pohlabeln H et al (2000) Occupational lung cancer risk for men in Germany: results from a pooled case-control study. Am J Epidemiol 151:384–395
Cocco P, Rice CH, Chen JQ, McCawley MA, McLaughlin JK, Dosemeci M (2001) Lung cancer risk, silica exposure, and silicosis in Chinese mines and pottery factories: the modifying role of other workplace lung carcinogens. Am J Ind Med 40:674–682. doi:10.1002/ajim.10022
Hughes JM, Weill H, Rando RJ, Shi R, McDonald AD, McDonald JC (2001) Cohort mortality study of North American industrial sand workers. II. Case-referent analysis of lung cancer and silicosis deaths. Ann Occup Hyg 45:201–207
Westberg HB, Bellander T (2003) Epidemiological adaptation of quartz exposure modeling in Swedish aluminum foundries: nested case-control study on lung cancer. Appl Occup Environ Hyg 18:1006–1013. doi:10.1080/10473220390244676
McDonald JC, McDonald AD, Hughes JM, Rando RJ, Weill H (2005) Mortality from lung and kidney disease in a cohort of North American industrial sand workers: an update. Ann Occup Hyg 49:367–373. doi:10.1093/annhyg/mei001
Cassidy A, Mannetje A, Van Tongeren M et al (2007) Occupational exposure to crystalline silica and risk of lung cancer: a multicenter case-control study in Europe. Epidemiology 18:36–43. doi:10.1097/01.ede.0000248515.28903.3c
Chen W, Bochmann F, Sun Y (2007) Effects of work related confounders on the association between silica exposure and lung cancer: a nested case–control study among Chinese miners and pottery workers. Int Arch Occup Environ Health 80:320–326. doi:10.1007/s00420-006-0137-0
Blettner M, Sauerbrei W, Schlehofer B, Scheuchenpflug T, Friedenreich C (1999) Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int J Epidemiol 28:1–9. doi:10.1093/ije/28.1.1
Blair A, Burg J, Foran J et al (1995) Guidelines for application of meta-analysis in environmental epidemiology. ISLI Risk Science Institute. Regul Toxicol Pharmacol 22:189–197. doi:10.1006/rtph.1995.1084
Berlin JA (1995) Invited commentary: benefits of heterogeneity in meta-analysis of data from epidemiologic studies. Am J Epidemiol 142:383–387
Colditz GA, Burdick E, Mosteller F (1995) Heterogeneity in meta-analysis of data from epidemiologic studies: a commentary. Am J Epidemiol 142:371–382
Jurek AM, Maldonado G, Greenland S, Church TR (2006) Exposure-measurement error is frequently ignored when interpreting epidemiologic study results. Eur J Epidemiol 21:871–876. doi:10.1007/s10654-006-9083-0
Dahmann D, Taeger D, Kappler M et al (2007) Assessment of exposure in epidemiological studies: the example of silica dust. J Expo Sci Environ Epidemiol 18:452–461. doi:10.1038/sj.jes.7500636
Wang W (2004) Nonparametric estimation of the Sojourn time distributions for a multi-path model. J R Stat Soc Ser B 65:921–936
Gordon I, Boffetta P, Demers PA (1998) A case study comparing a meta-analysis and a pooled analysis of studies of sinonasal cancer among wood workers. Epidemiology 9:518–524. doi:10.1097/00001648-199809000-00008
Department of Health and Human Services, Centers for Disease Control and Prevention. National Institute for Occupational Safety and Health (2002) NIOSH hazard review: HEALTH effects of occupational exposure to respirable crystalline silica. http://www.cdc.gov/niosh/02-129pd.html
Acknowledgments
We thank Dr. Marc Baril from the Quebec Research Institute for Occupational Health and Safety (Institut de recherche Robert-Sauvé en santé et en sécurité du travail—IRSST) for his support during all steps of this project. We also acknowledge the contributions of Hélène Girard and Jocelyne Bellemare, respectively, technician in documentation and librarian at Laval Hospital.
Source of funding:
Institut de recherche Robert-Sauvé en santé et en sécurité du travail (IRSST), Grant 99-163.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendices
Appendix 1: statistical methods for meta-analysis
Let I = 1,…, I indexes independent studies and J = 1,…, n i indexes exposure levels within studies. Let Y ij = log(RR ij ) be the estimated log risk ratio corresponding to exposure level x ij . The fixed-effects model is then written as
where f is a smooth continuous function, describing the relationship between the exposure level and the log relative risk response. ε ij → N(0, s 2 ij ) are the sampling errors in Y ij since the latter are estimated rather than observed. Estimated s 2 ij are given by individual studies. Finally, e ij → N(0, σ2) are the overall error terms. It is assumed that ε ij and e ij are independent.
For the mixed-effects model, a random effect term γ i common to points of the same study is added. This yield
where γ i → N(0,τ2) and are independent from ε ij and e ij . Testing heterogeneity corresponds them to performing the following:
The null hypothesis H0 is rejected at level α if
where χ 21,α satisfies \( P\left( {\chi_{1}^{2} > \chi_{1,\alpha }^{2} } \right) = \alpha. \) In both models, f is left completely unspecified providing flexibility. It is nonparametically estimated by a spline of order 3 [17]:
where (x − k i )+ = max(x − k i ,0) is the positive part and \( \left\{ {k_{i} ,\;i = 1, \ldots ,C - 1} \right\} \) are knots. We set C = 3. The models parameters \( \left\{ {\beta_{0 1} ,\beta_{0 2} ,\beta_{0 3} ,\beta_{ 1 3} ,\beta_{ 2 3,} \tau^{ 2} ,\sigma^{ 2} } \right\} \) are estimated by the algorithm given by Stram [19].
Rights and permissions
About this article
Cite this article
Lacasse, Y., Martin, S., Gagné, D. et al. Dose–response meta-analysis of silica and lung cancer. Cancer Causes Control 20, 925–933 (2009). https://doi.org/10.1007/s10552-009-9296-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-009-9296-0