Abstract
Purpose
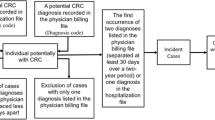
Claims data may be a suitable source studying associations between drugs and cancer. However, linkage between cancer registry and claims data including pharmacy-dispensing information is not always available. We examined the accuracy of claims-based definitions of incident cancers and their date of diagnosis.
Methods
Four claims-based definitions were developed to identify incident leukemia, lymphoma, lung, colorectal, stomach, and breast cancer. We identified a cohort of subjects aged ≥65 (1997–2000) from Pennsylvania Medicare and drug benefit program data linked with the state cancer registry. We calculated sensitivity, specificity, and positive predictive values of the claims-based definitions using registry as the gold standard. We further assessed the agreement between diagnosis dates from two data sources.
Results
All definitions had very high specificity (≥98%), while sensitivity varied between 40% and 90%. Test characteristics did not vary systematically by age groups. The date of first diagnosis according to Medicare data tended to be later than the date recorded in the registry data except for breast cancer. The differences in dates of first diagnosis were within 14 days for 75% to 88% of the cases. Bias due to outcome misclassification of our claims-based definition of cancer was minimal in our example of a cohort study.
Conclusions
Claims data can identify incident hematologic malignancies and solid tumors with very high specificity with sufficient agreement in the date of first diagnosis. The impact of bias due to outcome misclassification and thus the usefulness of claims-based cancer definitions as cancer outcome markers in etiologic studies need to be assessed for each study setting.


Similar content being viewed by others
Reference
Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM (2002) Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum 46(12):3151–3158
Setoguchi S, Solomon DH, Weinblatt ME et al (2006) Tumor necrosis factor alpha antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum 54(9):2757–2764
Warren JL, Feuer E, Potosky AL, Riley GF, Lynch CF (1999) Use of Medicare hospital and physician data to assess breast cancer incidence.[comment]. Medical Care 37(5):445–456
Cooper GS, Yuan Z, Stange KC, Dennis LK, Amini SB, Rimm AA (1999) The sensitivity of Medicare claims data for case ascertainment of six common cancers.[comment]. Medical Care 37(5):436–444
Solin LJ, Legorreta A, Schultz DJ, Levin HA, Zatz S, Goodman RL (1994) Analysis of a claims database for the identification of patients with carcinoma of the breast. J Med Syst 18(1):23–32
Solin LJ, MacPherson S, Schultz DJ, Hanchak NA (1997) Evaluation of an algorithm to identify women with carcinoma of the breast. J Med Syst 21(3):189–199
Leung KM, Hasan AG, Rees KS, Parker RG, Legorreta AP (1999) Patients with newly diagnosed carcinoma of the breast: validation of a claim-based identification algorithm. J Clin Epidemiol 52(1):57–64
Freeman JL, Zhang D, Freeman DH, Goodwin JS (2000) An approach to identifying incident breast cancer cases using Medicare claims data. J Clin Epidemiol 53(6):605–614
Penberthy L, McClish D, Manning C, Retchin S, Smith T (2005) The added value of claims for cancer surveillance: results of varying case definitions. Med Care 43(7):705–712
The North American Association of Central Cancer Registries, Inc. Official website (2004) (Accessed September, 9, 2005, at http://www.naaccr.org/.)
Fletcher RW, Fletcher SW (2005) Clinical Epidemiology: The Essentials. 4th edn. Lippincott Williams & Wilkins (ed)
Rothman KJ, Greenland S (1998) Modern Epidemiology. 2nd edn. Lippincott Williams & Wilkins
SEER Fast Stats (Accessed January 15, 2005, 2005, at http://seer.cancer.gov.)
Brenner H, Gefeller O (1993) Use of the positive predictive value to correct for disease misclassification in epidemiologic studies. Am J Epidemiol 138(11):1007–1015
Fanning J, Gangestad A, Andrews SJ (2000) National cancer data base/surveillance epidemiology and end results: potential insensitive-measure bias. Gynecol Oncol 77(3):450–453
Penberthy L, McClish D, Pugh A, Smith W, Manning C, Retchin S (2003) Using hospital discharge files to enhance cancer surveillance. Am J Epidemiol 158(1):27–34
Stang A, Glynn RJ, Gann PH, Taylor JO, Hennekens CH (1999) Cancer occurrence in the elderly: agreement between three major data sources. Ann Epidemiol 9(1):60–67
Wang PS, Walker AM, Tsuang MT, Orav EJ, Levin R, Avorn J (2001) Finding incident breast cancer cases through US claims data and a state cancer registry. Cancer Causes & Control 12(3):257–265
McClish DK, Penberthy L, Whittemore M et al (1997) Ability of Medicare claims data and cancer registries to identify cancer cases and treatment. Am J Epidemiol 145(3):227–233
Acknowledgments
The authors thank Jeffrey Peppercorn, MD, MPH, Assistant Professor of Medicine, Division of Hematology/Oncology, University of North Carolina at Chapel Hill for useful comments on the health care utilization data-based definitions for incident cancers and the draft version of this work.
The authors also thank Helen Mogun, MS for her contributions to linking the data of this work.
Financial Support: This project was support by a grant from the Engalitcheff Arthritis Outcomes Initiatives, Arthritis Foundation. Dr. Schneeweiss received support from the National Institute on Aging (RO1-AG021950, RO1- AG023178) and the Agency for Healthcare Research and Quality (2-RO1-HS10881), Department of Health and Human Services, Rockville, MD, and is principal investigator of the BWH DEcIDE Research Center funded by the Agency for Healthcare Research and Quality.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Setoguchi, S., Solomon, D.H., Glynn, R.J. et al. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between medicare claims and cancer registry data. Cancer Causes Control 18, 561–569 (2007). https://doi.org/10.1007/s10552-007-0131-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-007-0131-1