Abstract
Purpose
The aim of this study was to analyze the prevalence of breast cancer in relation to body height and to investigate associations between body height and breast cancer in Germany.
Methods
This retrospective cohort study included 135,741 adult women followed in one of 161 gynecology practices in Germany between January 2019 and December 2021. The 3 year prevalence of breast cancer (ICD-10: C50) during the study period was shown in relation to body height, which was included in this study as a five-category variable for women: ≤ 160 cm, 161–165 cm, 166–170 cm, 171–175 cm, > 175 cm. The associations between height and breast cancer were analyzed using logistic regression models adjusted for age and BMI.
Results
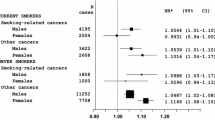
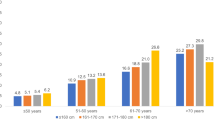
The prevalence of breast cancer increased from 5.1% in women ≤ 160 cm to 6.8% in women > 175 cm in the age group 51–60, and from 9.2% in women ≤ 160 cm to 12.2% in women 171–175 cm in the age group > 60 years. The OR for breast cancer was 1.18 (95% CI 1.12–1.24) for every 10 cm increase in height. Compared to height ≤ 160 cm, the OR for height 166–170 cm was 1.26 (1.15–1.39), for 171–175 cm 1.43 (1.27–1.61), and for > 175 cm 1.49 (1.28–1.74).
Conclusion
The results of this study suggest that greater body height in women is significantly related to an increased breast cancer risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Female breast cancer (BC) is the most commonly diagnosed cancer worldwide with approximately 2,3 million new cases per year [1]. Recent epidemiological data reveal an incidence of approximately 69,000 BC cases per year in Germany and BC is therefore also considered the most common cancer type in women in Germany [2]. However, there are numerous risk factors that can influence the odds of developing BC. These can be divided into modifiable (e.g., drinking alcohol, being overweight, not being physically active) and non-modifiable (e.g., older age, female sex, genetic mutations, height) [3]. Significantly, decades of epidemiological studies have focused largely on the association between overweight and BC, while the effect of height on BC risk has received far less attention [4,5,6,7]. In the past, a number of publications have shown that non-modifiable anthropometric factors such as height can influence the risk for several cancer sites such that tall people have an increased risk of cancer, although the results are inconsistent to some extent [4, 8,9,10]. In a large prospective UK cohort study including 1,297,124 women without previous cancer, subjects were followed up for cancer incidence and other confounding and modifying factors. In this study it turned out that the relative risk (RR) adjusted for multiple variables such as BMI and socioeconomic status for cancer overall was 1.16 (95% CI 1.14–1.17; p < 0.0001) for every 10 cm increase in height. In total, the height-related RRs increased significantly for ten assessed cancer sites (e.g., malignant melanoma, breast cancer, ovarian cancer, and endometrial cancer) [11].
However, there is a lack of evidence with regard to the relationship between body height and BC risk in Germany. Given that the range of height in each population is relatively small, large patient cohorts are needed to obtain reliable results. In response to this need, we report here a large retrospective study with 135,741 women in Germany followed in one of 161 gynecology practices in Germany between January 2019 and December 2021 to analyze the prevalence of BC in relation to body height and to investigate associations between height and BC adjusted for age and BMI.
Methods
Database
This study used data from the Disease Analyzer database (IQVIA). This database has already been described extensively in the literature [12]. To summarize, the Disease Analyzer database contains demographic, diagnosis, and prescription data from patients followed in general and specialized practices in Germany. Practices are selected for inclusion in the database based on multiple factors (i.e., specialty group, community size category, and German federal state), and the database is composed of around 3–5% of all practices in Germany. Diagnosis and prescription data are coded using the International Classification of Diseases, 10th revision (ICD-10), and the Anatomical Classification of Pharmaceutical Products of the European Pharmaceutical Marketing Research Association (EphMRA), respectively. It has previously been shown that the panel of practices included in the Disease Analyzer database is representative of general and specialized practices in Germany [12]. Finally, this database has already been used in previous studies focusing on BC [13, 14].
Study population
This retrospective cohort study included 135,741 adult women followed in 161 gynecology practices in Germany between January 2019 and December 2021. The only inclusion criterion was at least one documented height value during this period. Height values were available for 135,741 (19.3%) out of 702,475 women.
Study outcomes and variables
The outcome of the study was the prevalence of BC (ICD-10: C50) diagnoses during the study period as a function of height. Height was included in this study as a five-category variable for women: ≤ 160 cm, 161–165 cm, 166–170 cm, 171–175 cm, > 175 cm.
Statistical analyses
Age at first visit in 2019–2021 was compared between height categories. As there was a strong relationship between height and age (taller women were younger), all analyses were either performed by age group or adjusted for age. First, the 3 years prevalence of BC was descriptively shown. Then, the associations between height and BC were analyzed using logistic regression models adjusted for age and BMI. The results of the regression analyses are displayed as odds ratios (ORs) and 95% confidence intervals (95% CI). In the first model, ORs showed the risk increase for each height category compared to ≤ 160 cm. In the second model, ORs showed how the risk of BC increased for every 10 cm increase in height. P-values lower than 0.05 were considered statistically significant. Analyses were conducted with SAS 9.4 (SAS Institute, Cary, US).
Results
This study included 135,741 women with an average age of 39.8 years (SD 15.2). The average body height was 166.4 cm and the average BMI was 26.0 kg/m2 (Table 1). Figure 1 shows the prevalence of BC by age group and height category. The prevalence increased from 5.1% in women ≤ 160 cm to 6.8% in women > 175 cm in the age group 51–60, and from 9.2% in women ≤ 160 cm to 12.2% in women 171–175 cm in the age group > 60 years. The results of the age- and BMI-adjusted logistic regression analyses are displayed in Table 2. The OR for BC was 1.18 (95% CI 1.12–1.24) for every 10 cm increase in height. Compared to height ≤ 160 cm, the OR for height 166–170 cm was 1.26 (1.15–1.39), for 171–175 cm 1.43 (1.27–1.61), and for > 175 cm 1.49 (1.28–1.74) (Table 2).
Discussion
In this large retrospective study carried out in Germany, we identified a trend of increasing BC prevalence with increasing body height and found a highly significant positive association between body height and BC risk using multivariable logistic regression models adjusted for age and body mass index.
In general, the significant positive association between body height and BC risk is in line with numerous epidemiological studies published previously [15,16,17,18,19,20,21]. More recently, a large meta-analysis conducted by Zhang et al. was performed to investigate associations between height and BC risk using data from 159 prospective cohort studies performed in several countries (e.g., USA, Canada, Sweden, and Norway). They showed that the pooled RR for developing BC was 1.17 (95% confidence interval CI 1.15–1.19) per 10 cm increase in height. The authors also analyzed height-related genetic variants (single-nucleotide polymorphisms, SNPs) and determined that eight genetic variants were associated with an increased BC cancer risk. Further Mendelian randomization analysis reveals an odds ratio of 1.22 (95% CI 1.13–1.32) for BC per 10 cm increase in genetically predicted height and provides strong evidence that the association between adult height and BC risk is likely to be causal [22].
Although height is non-modifiable for the individual, it should be mentioned that final body height is the result of various genetic and environmental factors occurring before birth and during childhood and adulthood, and there is growing evidence that these factors (e.g., childhood diet and nutritional status) can also influence cancer risk in adulthood [23, 24]. In particular, biological pathways such as insulin-like growth factor-1 (IGF-1) signaling are involved in both adult body height and carcinogenesis and therefore considered a possible causal link regarding height and cancer risk [25, 26]. Notably, all factors of the IGF-1 system, including IGF-1, IGF-binding proteins (IGFBPs), and the IGF-1 receptor (IGF-1R) play a pivotal role in BC development, progression, and metastasis [27,28,29]. Large prospective population studies from many different countries have shown an increasing BC risk with increasing serum levels of IGF-1. This BC risk related to IGF-1 level was highly significant for premenopausal women only, indicating the possible importance of IGF-1 levels in early life or with respect to an influence on mammary gland development in women [30,31,32,33]. Conversely, in another prospective study it was shown that mutations in the growth hormone receptor (GHR) gene lead to reduced IGF-1 levels, which are associated with severe short stature in the subjects concerned and significantly reduced diabetes and BC risks [34]. However, the exact reason for the increased BC risk with increasing body height remains unclear and further research is necessary to uncover the underlying mechanisms.
According to the literature, analyzing the association between body height and cancer risk requires adjustments for potential confounding factors to achieve reliable results (Table 1). For instance, the association between BC risk and overweight varies according to menopausal status [35, 36]. In particular, obese women have a reduced risk of hormone receptor (HR)-positive premenopausal BC and an increased risk of HR-positive postmenopausal BC compared to women with a normal BMI, whereby the precise mechanism of these paradoxical effects remains elusive [5, 37, 38]. It seems clear that among premenopausal women, overweight leads to anovulation and lower estrogen levels while adipose tissue in obese postmenopausal women produces considerable amounts of estrogen, leading to an increased BC risk [39]. As is well-known for other diseases, age is the most important non-modifiable risk factor for BC. The BC incidence in Germany is relatively low before the age of 30 (< 50 per 100,000 women) but increases strongly until the age of 65 (300 per 100,000 women) [40, 41]. However, after adjustment for these possible confounding factors, we were able to present reliable data for an increased BC risk with increasing body height in this large retrospective study of women in Germany.
Strengths and limitations
Our retrospective cohort study has several strengths: The German Disease Analyzer (DA) is a large European outpatient database containing data from 161 gynecological practices in Germany. The representativeness of the diagnoses it contains has already been validated [12]. Furthermore, a large patient cohort (135,741 women) was used for this study and to avoid confounding factors, adjustment for age and BMI was performed.
However, the study results should be interpreted in the light of several limitations: The DA does not contain information on external confounding factors (e.g., alcohol, tobacco consumption, socioeconomic status) and body height was only available for 16% of all patients. Moreover, the average BMI (26.0 kg/m2) of women within the study indicates that tendentially more overweight women are part of the study population. In addition, there is a lack of detailed information regarding the molecular subtype of BC, TNM classification, menopausal status, and other covariates such as hormone replacement therapy (HRT).
Finally, this study is a retrospective database analysis that does not allow conclusions to be drawn about causal relationships.
Data availability
Anonymized raw data are available on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020—GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Kaatsch P, Spix C, Katalinic A (2018) Cancer in Germany in 2013/2014. Robert Koch Institute, Berlin
Lukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanislawek A (2021) Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers (Basel). https://doi.org/10.3390/cancers13174287
Choi YJ, Lee DH, Han KD, Yoon H, Shin CM, Park YS, Kim N (2019) Adult height in relation to risk of cancer in a cohort of 22,809,722 Korean adults. Br J Cancer 120(6):668–674. https://doi.org/10.1038/s41416-018-0371-8
Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A (2014) Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev 36:114–136. https://doi.org/10.1093/epirev/mxt010
Suzuki R, Orsini N, Saji S, Key TJ, Wolk A (2009) Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status—a meta-analysis. Int J Cancer 124(3):698–712. https://doi.org/10.1002/ijc.23943
Vrieling A, Buck K, Kaaks R, Chang-Claude J (2010) Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status—a meta-analysis. Breast Cancer Res Treat 123(3):641–649. https://doi.org/10.1007/s10549-010-1116-4
Batty GD, Shipley MJ, Langenberg C, Marmot MG, Davey Smith G (2006) Adult height in relation to mortality from 14 cancer sites in men in London (UK)—evidence from the original whitehall study. Ann Oncol 17(1):157–166. https://doi.org/10.1093/annonc/mdj018
Sung J, Song YM, Lawlor DA, Smith GD, Ebrahim S (2009) Height and site-specific cancer risk—a cohort study of a Korean adult population. Am J Epidemiol 170(1):53–64. https://doi.org/10.1093/aje/kwp088
Batty GD, Barzi F, Woodward M, Jamrozik K, Woo J, Kim HC, Ueshima H, Huxley RR, Asia Pacific Cohort Studies Collaboration (2010) Adult height and cancer mortality in Asia—the Asia Pacific Cohort Studies Collaboration. Ann Oncol 21(3):646–654. https://doi.org/10.1093/annonc/mdp363
Green J, Cairns BJ, Casabonne D, Wright FL, Reeves G, Beral V, Million Women Study collaborators (2011) Height and cancer incidence in the million women study—prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol 12(8):785–794. https://doi.org/10.1016/S1470-2045(11)70154-1
Rathmann W, Bongaerts B, Carius HJ, Kruppert S, Kostev K (2018) Basic characteristics and representativeness of the German disease analyzer database. Int J Clin Pharmacol Ther 56(10):459–466. https://doi.org/10.5414/CP203320
Stumpf U, Kostev K, Kyvernitakis J, Bocker W, Hadji P (2019) Incidence of fractures in young women with breast cancer—a retrospective cohort study. J Bone Oncol 18:100254. https://doi.org/10.1016/j.jbo.2019.100254
Jacob L, Kostev K, Kalder M (2020) Prescription of hormone replacement therapy prior to and after the diagnosis of gynecological cancers in German patients. J Cancer Res Clin Oncol 146(6):1567–1573. https://doi.org/10.1007/s00432-020-03185-y
Okasha M, McCarron P, Gunnell D, Smith GD (2003) Exposures in childhood, adolescence and early adulthood and breast cancer risk—a systematic review of the literature. Breast Cancer Res Treat 78(2):223–276. https://doi.org/10.1023/a:1022988918755
Friedenreich CM (2001) Review of anthropometric factors and breast cancer risk. Eur J Cancer Prev 10(1):15–32. https://doi.org/10.1097/00008469-200102000-00003
Tornberg SA, Holm LE, Carstensen JM (1988) Breast cancer risk in relation to serum cholesterol, serum beta-lipoprotein, height, weight, and blood pressure. Acta Oncol 27(1):31–37. https://doi.org/10.3109/02841868809090315
Tretli S (1989) Height and weight in relation to breast cancer morbidity and mortality a prospective study of 570,000 women in Norway. Int J Cancer 44(1):23–30. https://doi.org/10.1002/ijc.2910440105
Vatten LJ, Kvinnsland S (1992) Prospective study of height, body mass index and risk of breast cancer. Acta Oncol 31(2):195–200. https://doi.org/10.3109/02841869209088902
Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TI (2004) Growth patterns and the risk of breast cancer in women. N Engl J Med 351(16):1619–1626. https://doi.org/10.1056/NEJMoa040576
White KK, Park SY, Kolonel LN, Henderson BE, Wilkens LR (2012) Body size and breast cancer risk—the multiethnic cohort. Int J Cancer 131(5):E705–E716. https://doi.org/10.1002/ijc.27373
Zhang B, Shu XO, Delahanty RJ, Zeng C, Michailidou K, Bolla MK, Wang Q, Dennis J, Wen W, Long J, Li C, Dunning AM, Chang-Claude J, Shah M, Perkins BJ et al (2015) Height and breast cancer risk—evidence from prospective studies and mendelian randomization. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv219
Whitley E, Martin RM, Smith GD, Holly JM, Gunnell D (2009) Childhood stature and adult cancer risk—the boyd orr cohort. Cancer Causes Control 20(2):243–251. https://doi.org/10.1007/s10552-008-9239-1
Silventoinen K (2003) Determinants of variation in adult body height. J Biosoc Sci 35(2):263–285. https://doi.org/10.1017/s0021932003002633
Endogenous H, Breast Cancer Collaborative G, Key TJ, Appleby PN, Reeves GK, Roddam AW (2010) Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk—pooled individual data analysis of 17 prospective studies. Lancet Oncol 11(6):530–542. https://doi.org/10.1016/S1470-2045(10)70095-4
Rogers I, Metcalfe C, Gunnell D, Emmett P, Dunger D, Holly J, Avon Longitudinal Study of Parents Children Study Team (2006) Insulin-like growth factor-I and growth in height, leg length, and trunk length between ages 5 and 10 years. J Clin Endocrinol Metab 91(7):2514–2519. https://doi.org/10.1210/jc.2006-0388
Christopoulos PF, Msaouel P, Koutsilieris M (2015) The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer 14:43. https://doi.org/10.1186/s12943-015-0291-7
Becker MA, Ibrahim YH, Cui X, Lee AV, Yee D (2011) The IGF pathway regulates ERalpha through a S6K1-dependent mechanism in breast cancer cells. Mol Endocrinol 25(3):516–528. https://doi.org/10.1210/me.2010-0373
Sarfstein R, Pasmanik-Chor M, Yeheskel A, Edry L, Shomron N, Warman N, Wertheimer E, Maor S, Shochat L, Werner H (2012) Insulin-like growth factor-I receptor (IGF-IR) translocates to nucleus and autoregulates IGF-IR gene expression in breast cancer cells. J Biol Chem 287(4):2766–2776. https://doi.org/10.1074/jbc.M111.281782
Pollak MN, Schernhammer ES, Hankinson SE (2004) Insulin-like growth factors and neoplasia. Nat Rev Cancer 4(7):505–518. https://doi.org/10.1038/nrc1387
Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M (1998) Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 351(9113):1393–1396. https://doi.org/10.1016/S0140-6736(97)10384-1
Toniolo P, Bruning PF, Akhmedkhanov A, Bonfrer JM, Koenig KL, Lukanova A, Shore RE, Zeleniuch-Jacquotte A (2000) Serum insulin-like growth factor-I and breast cancer. Int J Cancer 88(5):828–832. https://doi.org/10.1002/1097-0215(20001201)88:5%3c828::aid-ijc22%3e3.0.co;2-8
Kaaks R, Lundin E, Rinaldi S, Manjer J, Biessy C, Soderberg S, Lenner P, Janzon L, Riboli E, Berglund G, Hallmans G (2002) Prospective study of IGF-I, IGF-binding proteins, and breast cancer risk, in northern and southern Sweden. Cancer Causes Control 13(4):307–316. https://doi.org/10.1023/a:1015270324325
Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD (2011) Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 3(70):70ra13. https://doi.org/10.1126/scitranslmed.3001845
Amadou A, Ferrari P, Muwonge R, Moskal A, Biessy C, Romieu I, Hainaut P (2013) Overweight, obesity and risk of premenopausal breast cancer according to ethnicity—a systematic review and dose-response meta-analysis. Obes Rev 14(8):665–678. https://doi.org/10.1111/obr.12028
Garcia-Estevez L, Cortes J, Perez S, Calvo I, Gallegos I, Moreno-Bueno G (2021) Obesity and breast cancer—a paradoxical and controversial relationship influenced by menopausal status. Front Oncol 11:705911. https://doi.org/10.3389/fonc.2021.705911
Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO (2001) Overweight as an avoidable cause of cancer in Europe. Int J Cancer 91(3):421–430. https://doi.org/10.1002/1097-0215(200002)9999:9999%3c::aid-ijc1053%3e3.0.co;2-t
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer—a systematic review and meta-analysis of prospective observational studies. Lancet 371(9612):569–578. https://doi.org/10.1016/S0140-6736(08)60269-X
Zhang X, Tworoger SS, Eliassen AH, Hankinson SE (2013) Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res Treat 137(3):883–892. https://doi.org/10.1007/s10549-012-2391-z
Kluttig A, Schmidt-Pokrzywniak A (2009) Established and suspected risk factors in breast cancer aetiology. Breast Care (Basel) 4(2):82–87. https://doi.org/10.1159/000211368
Barnes B, Kraywinkel K, Nowossadeck E, Schönfeld I, Starker A, Wienecke A, Wolf U (2016) Bericht zum Krebsgeschehen in Deutschland 2016. Robert Koch-Institut, Berlin
Funding
Open Access funding enabled and organized by Projekt DEAL. N.G. was funded by the Stiftung P. E. Kempkes (Grant No. 01/2021) and supported by a Research Grant of the University Medical Center Giessen and Marburg (UKGM, Grant No. 03/2022 MR). In addition, NG was supported by the Clinician Scientist program (SUCCESS-program) of the Philipps-University and the University Hospital Giessen and Marburg (UKGM).
Author information
Authors and Affiliations
Contributions
NG: managed the literature searches, wrote the first draft of the manuscript, and corrected the manuscript. KK: performed the data analyses, contributed to the design of the study and corrected the manuscript. MK and SG: contributed to the design of the study and corrected the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The database used for this study includes only anonymized data in compliance with the provisions set forth in the applicable data protection laws. German law allows the use of anonymous electronic medical records for research purposes under certain conditions. In accordance with this legislation, it is not necessary to obtain informed consent from patients or approval from a medical ethics committee for this type of observational study that contains no directly identifiable data. Because patients were only queried as aggregates and no protected health information was available for queries, no Institutional Review Board approval was required for the use of this database or the completion of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gremke, N., Griewing, S., Kalder, M. et al. Positive association between body height and breast cancer prevalence: a retrospective study with 135,741 women in Germany. Breast Cancer Res Treat 196, 349–354 (2022). https://doi.org/10.1007/s10549-022-06730-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06730-0