Abstract
Purpose
Guidelines recommend endocrine treatment for estrogen receptor-positive (ER+) breast cancers for up to 10 years. Earlier data suggest that the 70-gene signature (MammaPrint) has potential to select patients that have an excellent survival without chemotherapy and limited or no tamoxifen treatment. The aim was to validate the 70-gene signature ultralow-risk classification for endocrine therapy decision making.
Methods
In the IKA trial, postmenopausal patients with non-metastatic breast cancer had been randomized between no or limited adjuvant tamoxifen treatment without receiving chemotherapy. For this secondary analysis, FFPE tumor material was obtained of ER+HER2− patients with 0–3 positive lymph nodes and tested for the 70-gene signature. Distant recurrence-free interval (DRFI) long-term follow-up data were collected. Kaplan–Meier curves were used to estimate DRFI, stratified by lymph node status, for the three predefined 70-gene signature risk groups.
Results
A reliable 70-gene signature could be obtained for 135 patients. Of the node-negative and node-positive patients, respectively, 20% and 13% had an ultralow-risk classification. No DRFI events were observed for node-negative patients with an ultralow-risk score in the first 10 years. The 10-year DRFI was 90% and 66% in the low-risk (but not ultralow) and high-risk classified node-negative patients, respectively.
Conclusion
These survival analyses indicate that the postmenopausal node-negative ER+HER2− patients with an ultralow-risk 70-gene signature score have an excellent 10-year DRFI after surgery with a median of 1 year of endocrine treatment. This is in line with published results of the STO-3-randomized clinical trial and supports the concept that it is possible to reduce the duration of endocrine treatment in selected patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Endocrine therapy is a key element of adjuvant systemic treatment for patients with estrogen receptor-positive (ER+) breast cancer, and guidelines recommending endocrine therapy for up to 10 years [1]. Five years of tamoxifen reduces 10-year breast cancer mortality rates by approximately 25% (proportionally) compared with no endocrine therapy, while this is 40% (proportionally) for five years of an aromatase inhibitor (AI) compared to no endocrine therapy [2]. The absolute 15-year breast cancer mortality reduction of 5 years of adjuvant tamoxifen versus nil is 9.2% [3]. Extension of adjuvant endocrine therapy duration for up to 10 years further reduces recurrence risk, with hazard rates of several randomized controlled trials varying between 0.57 and 1.0, depending on tamoxifen and aromatase inhibitor sequence, total endocrine therapy duration in the control arm, and case mix of the population under study [1]. Survival benefits have been observed for 10 versus 5 years of tamoxifen and for 5 years of AI therapy after 5 years of tamoxifen [1]. The absolute 15-year breast cancer mortality reduction for 10 versus 5 years of tamoxifen is 2.8% [4]. Longer follow-up of the extended-AI therapy trials will answer the question of whether longer AI therapy duration will translate into a survival benefit [1].
Although endocrine therapy toxicities are rarely life threatening, side effects are common and are leading to non-adherence which in turn can lead to a higher recurrence risk. Non-adherence is more frequent in women experiencing a moderate to high impact of the side effects on their daily lives [5, 6]. Common side effects include hot flashes, muscle and joint pain, weight gain, fatigue, mood swings, difficulty concentrating, numbness or tingling in the extremities, vaginal dryness, and hair loss. Low self-esteem and low libido are perhaps more a consequence of the above-mentioned side effects but, if present, can have a considerable impact on patients’ lives [5, 6]. Rare, life-threatening tamoxifen-related side effects include thromboembolic complications and, restricted to postmenopausal women, an increased endometrial cancer risk [7]. AI-specific side effects are osteoporosis with increased fracture risk, and increased odds of cardiovascular events [2, 8].
A test identifying patients who have an excellent prognosis with only a limited duration of endocrine therapy would allow us to reduce overtreatment and improve patients’ quality of life. On the other hand, such a test might help increase treatment adherence in patients for whom longer endocrine treatment is required to substantially increase the odds of a good outcome.
The Food & Drug Administration (FDA)-cleared 70-gene signature MammaPrint was originally developed to identify patients who have a low-risk of distant recurrence and cancer-related death and who may be candidates for (neo)adjuvant chemotherapy de-escalation [9,10,11].
More recently, a threshold identifying an ultralow-risk group was developed on the same 70-gene signature and validated in a post hoc analysis of patients from the STO-3 trial. The ultralow-risk group consists of patients with indolent tumors and excellent prognosis up to 20 years after diagnosis [12, 13]. The STO-3 trial included node-negative patients, with tumors up to 30 mm, who did not receive chemotherapy and only limited (2 or 5 years) or no tamoxifen treatment. The 15% of patients in the STO-3 study with an ultralow-risk classification had a 20-year breast cancer-specific survival of 97% with limited endocrine treatment and 94% without any endocrine treatment, with no statistically significant difference between the two groups.
These data can have a high impact and help de-escalation of endocrine treatment in a substantial group of breast cancer patients; therefore, we set out to validate these data in an independent dataset. The Dutch IKA trial dataset was used for the validation [14, 15], which consists of well-annotated, high-quality data and tumor tissue blocks of patients treated with no or only a short duration of adjuvant endocrine therapy and 20 years of follow-up available.
Our goal was to validate the association of the 70-gene signature ultralow-risk classification with indolent behavior and excellent prognosis in patients with ER+HER2− invasive, node-negative, and node-positive breast cancer treated with 0–3 years of tamoxifen in the IKA-randomized clinical trial.
Methods
Patients and material
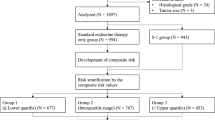
For the current analysis, we used a subset of the Dutch multicenter IKA-randomized clinical trial that enrolled patients from 1982 till 1994. The REMARK (Reporting Recommendations for Tumor Marker Prognostic Studies) criteria were used to report this study [16]. Postmenopausal, non-metastatic, breast cancer patients (N = 1662) had been randomized between no adjuvant endocrine therapy (control arm), 1 or 3 years of tamoxifen treatment. The patient characteristics for the full-study population have been presented previously, and clinical outcome data were part of the Oxford meta-analysis [14, 17, 18]. Based on an interim analysis in 1989, the study protocol was amended for all node-positive patients to receive at least one year of tamoxifen. None of the patients had received (neo)adjuvant chemotherapy, as was the standard at that time for postmenopausal patients. Only local therapy was mandatory (mastectomy/breast conserving surgery ± radiotherapy, or upfront curative radiotherapy without surgery for patients with tumor-positive sub-clavicular ipsilateral lymph nodes (N = 55/1662 (3.3%)).
Formalin-fixed paraffin-embedded (FFPE) tumor blocks were available for 739 patients who had received upfront surgery. Estrogen receptor alpha (ER), progesterone receptor (PR), HER2, Ki67, and mitotic activity index (MAI) were centrally assessed and have been reported previously [15, 18]. For the current analysis, stage I–III patients with an ER-positive, HER2-negative breast cancer were selected (N = 482). For 346 patients, sufficient tumor material was available to perform the 70-gene signature MammaPrint assay.
MammaPrint RNA analysis
FFPE tumor slides (5 × 5 µm) with a minimum of 30% tumor cells were sent to Agendia for standard RNA isolation (Qiagen RNeasy FFPE kit), and 70-gene signature MammaPrint and 80-gene BluePrint Molecular Subtype testing. This was done on a custom-designed Agilent microarray according to standard protocols and with previously described thresholds [19,20,21]. Agendia, blinded for clinical variables and outcomes, classified the samples for 70-gene signature into three groups: ultralow, low, or high risk, and for BluePrint into three subtypes: luminal, basal, or ERBB2/HER2-type. Agendia maintains a quality system in compliance with international regulations such as the FDA and the EU in vitro diagnostics directives.
Statistical analysis
All analyses were performed based on three 70-gene signature MammaPrint index (MPI) thresholds: ultralow risk (MPI > + 0.355), low risk (0 > MPI ≤ + 0.355), and high risk (MPI ≤ 0) [22]. Kaplan–Meier analyses were performed for recurrence-free interval (RFI), distant recurrence-free interval (DRFI), and breast cancer-specific survival (BCSS) according to the DATECAN definitions [23]. Survival distributions were compared using the log-rank test. In a multivariable cox proportional hazard model adjusting for age, PR, T-stage, grade, allocated treatment arm, and stratified for nodal status and study protocol version, the hazard ratios per 70-gene signature risk group are calculated with the ultralow-risk as reference. A likelihood ratio test on the multivariable model predicting breast cancer-specific survival with or without 70-gene signature risk was used to test for added value to the known prognostic variables. For risk groups that have equal RFI and DRFI events, we report survival estimates as (D)RFI for simplicity. For all endpoints, patients were censored at diagnosis of a new primary cancer. In case of an ipsilateral tumor, local assessment was followed for the classification as recurrence or new primary tumor. Deaths with unknown cause after distant breast cancer recurrence were classified as BCSS events, similar to the approach taken in the Oxford Overview [17].
The survival tree developed using recursive partitioning in the STO-3 trial was also followed for the node-negative patients in the current study, resulting in four groups [13]. For the node-positive patients, a new decision tree was created using the rpart package (version 4.1–15) in R (version 3.6.1). The following input variables were used: 70-gene signature risk, age, Ki67, MAI, T-status, and grade. The final tree was selected with a tenfold cross validation.
Results
A 70-gene signature score was obtained for 135 ER+, HER2− samples, of which 34 patients received no adjuvant endocrine treatment at all (Fig. 1). 101 patients were randomized to receive 1 year (N = 53) or 3 years (N = 48) of adjuvant tamoxifen and no other systemic treatment for the primary tumor. Due to a change in randomization after the interim analysis (see methods), node-positive and larger tumors are enriched in the tamoxifen-treated arm (Table 1). No significant selection differences were detected between 135 patients with ER+HER2− tumors with a 70-gene signature result and the 347 patients with ER+HER2− tumors without a 70-gene signature result for the most important tumor and patient characteristics (Table 1).
Using the 70-gene signature, we classified 53 (40%), 59 (43%), and 23 (17%) of the patients as respectively high, low, and ultralow risk. In Table 2, the distributions of clinicopathological characteristics within the three 70-gene signature scores are shown. MAI, PR status, and grade were significantly different between the groups: In the ultralow-risk group, 13% (3/23) of the patients had a tumor with MAI ≥ 8/2 mm2, while this was 35% (20/57) and 70% (37/53) for the genomic low and high-risk group, respectively. The PR status was negative (< 10% positive staining) in 29% (6/21) of ultralow-risk tumors, while this was 44% (23/52) and 54% (27/50) for the genomic low-risk and high-risk tumors, respectively. Similarly, in the ultralow-risk group, 65% (15/23) of the tumors were grade I, while only 39% (23/59) and 13% (7/53) were grade I for the genomic low and high-risk tumors, respectively. Within the node-negative subset, 20% (16/80) patients had an ultralow-risk 70-gene signature result. All ultralow and low-risk patients had a Luminal classification by BluePrint, whereas 94% (50/53) of the high-risk tumors were classified as Luminal.
Survival analysis
Median follow-up was 27 years using the reverse Kaplan–Meier method [24]. In the node-negative patients with an ultralow-risk 70-gene signature score (N = 16), no (D)RFI events were observed in the first 10 years after diagnosis, while the 20-year (D)RFI was 82% [95% CI 61–100] (Table 3). For the 70-gene signature low-risk (but not ultralow) node-negative patients, the 10-year and 20-year (D)RFI were 90% [95% CI 79–100] for both time points. In the genomic high-risk node-negative patients, the 10-year (D)RFI was 66% [95% CI 51–86], and the 20-year (D)RFI was 61% [95% CI 45–83] (Table 3). The corresponding Kaplan–Meier plots for node-negative patients are shown in Fig. 2A (RFI) and Fig. 2C (DRFI). Breast cancer-specific survival is shown in Fig. 2E for the node-negative patients. The 10-year BCSS in node-negative patients is 100% for the ultralow-risk group, 93% [95% CI 84–100] for the low-risk group, and 72% [95% CI 58–91] for the 70-gene signature high-risk group. The 20-year BCSS in node-negative patients is respectively for ultralow, low, and high risk: 92% [95% CI 77–100], 93% [95% CI 84–100], and 60% [95% CI 43–85].
Kaplan–Meier plots of survival in node-negative and node-positive patients. Recurrence-Free Interval of the patients stratified based on 70-gene MammaPrint risk score for A node-negative patients and B node-positive patients. Distant Recurrence-Free Interval of the patients stratified based on 70-gene MammaPrint risk score for C node-negative patients and D node-positive patients. Breast cancer-specific survival of the patients stratified based on 70-gene MammaPrint risk score for E node-negative patients and F node-positive patients
In the node-positive patients with an ultralow-risk 70-gene signature score (N = 7), the (D)RFI was 69% [95% CI 40–100] at 10 and 20 years. The 70-gene signature low (but not ultralow) risk node-positive patients had a RFI of 79% [95% CI 63–100] for all time points with an almost similar DRFI of 78% [95% CI 61–100]. For the high-risk node-positive patients, the 10-year and 20-year RFI was 42% [95% CI 25–70] for both time points (Table 3; Fig. 2B) and the DRFI was 53% [95% CI 35–79] at 10 years and 45% [95% CI 27–75] at 20 years (Table 3; Fig. 2D). Breast cancer-specific survival is shown in in Fig. 2F for node-positive patients. The 20-year BCSS in the node-positive patients is 83% [95% CI 58–100] for the ultralow-risk group, 78% [95% CI 60–100] for the low-risk group, and 42% [95% CI 24–74] for the 70-gene signature high-risk group (Table 3). In Online Resource 1 and 2, the BCSS without censoring for the second primary tumor are shown. The results were similar to the results with censoring for second primary tumor (Fig. 2 and Table 3).
In the first 20 years, breast cancer patients with a 70-gene high-risk tumor had a significant higher risk of disease-specific death compared with ultralow-risk patients in a multivariable Cox analysis (hazard ratio 6.35 [95% CI 1.35–29.96], while for patient with a low-, but not ultralow-risk tumor, the hazard ratio was not significant different (hazard ratio 1.27 [95% CI 0.23–6.93]. A likelihood ratio test on a multivariable model predicting breast cancer-specific survival with or without 70-gene signature risk scores indicates that the 70-gene signature risk score has added value (∆LR-χ2 = 14.6, p < 0.001) to the know prognostic variables.
Survival decision tree
As suggested by the recursive partitioning survival tree by Esserman et al. [13], we divided the node-negative patients first on genomic ultralow-risk and second, for the genomic non-ultralow-risk patients, on size (≤ or > 20 mm, respectively, T1 or T2–3). The T1 not ultralow-risk patients are subdivided into 70-gene signature low and high risk. The sixteen ultralow-risk patients are discussed before, and in this group, one BCSS event (6%) occurred in the 20-year follow-up. In the thirteen T1 low-risk patients, no BCSS events occurred. In the seven T1 high-risk patients, two BCSS events (29%) occurred. In the 44 T2–3 not ultralow-risk patients, ten BCSS events (23%) occurred (Fig. 3A). The Kaplan–Meier survival plots for the different groups are shown in Fig. 3B (RFI) and 3C (BCSS).
Predefined decision tree for the node-negative patients and Kaplan–Meier Plots based on the risk prediction. A The decision tool was proposed by Esserman and filled in with the node-negative patients from the IKA tamoxifen trial resulting in four groups. Risk prediction, number of Breast Cancer-Specific Deaths at 10 and 20 years, risk group name and number of patients are shown for each group. B Kaplan–Meier plots of recurrence-free interval and C breast cancer-specific survival are shown for the four end groups of the decision tree: MammaPrint ultralow, T1 MammaPrint low risk, T1 MammaPrint high risk, T2-3 MammaPrint not ultralow
For the node-positive patients, a new survival tree was made (Fig. 4) with 70-gene signature as the most informative variable to predict BCSS compared to the other input variables: age, Ki67, MAI, T-status, and grade. The 70-gene signature low-risk (including ultralow) groups are further split by size. This resulted in no BCSS event for the seven patients with 70-gene signature low risk and tumor size ≤ 20 mm. In the 70-gene signature low risk and tumor size > 20 mm, 26 patients are grouped and in this group, five BCCS events occurred (19%). In the 70-gene signature high-risk patients, nine events are observed in 22 patients (41%).
Explorative decision tree for the node-positive patients and Kaplan–Meier Plots based on the risk prediction. The decision tool was developed using rpart with input variables: 70-gene MammaPrint classification (ultralow, low risk, or high risk), age, Ki67, MAI, tumor size, and grade. A The node-positive patients from the IKA tamoxifen trial were classified in three groups. Risk prediction, number of Breast Cancer-Specific Deaths at 10 and 20 years, risk group name, and number of patients are shown for each group. Note that the low-risk groups include ultralow-risk patients. B Kaplan–Meier plots recurrence-free interval and C breast cancer-specific survival shown for the three final groups of the decision tree: MammaPrint low risk with size ≤ 20 mm, MammaPrint low risk with size > 20 mm, and MammaPrint high risk
Discussion
In this post hoc analysis of a representative subset of postmenopausal, stage I-III patients with ER+HER2− tumor that participated in the randomized IKA trial of adjuvant tamoxifen versus nil, we observed an excellent outcome with no BCSS events during the first 10 years for node-negative patients with a ER+HER2− and 70-gene signature ultralow-risk tumor. These data confirm earlier findings for the ultralow-risk 70-gene signature result in the STO-3 trial [13] and are in line with the recent findings in the MINDACT series [25]. Importantly, in our studies, we did not only report on BCSS events but also on any type of breast cancer recurrence (RFI). Remarkably, node-negative, postmenopausal patients with ER+HER2− ultralow-risk tumors did not recur at all in the first 10 years of follow-up. On the other hand, our data also show that postmenopausal patients with ultralow-risk tumors still have a risk of recurrence after 10 years if treated with only 0–3 years of adjuvant tamoxifen. The mild discrepancy in the prognosis of the ultralow-risk patients between the IKA trial and the STO-3 trial after 10 years might be caused by noteworthy differences in the patient case mixes. Most importantly, the IKA trial was not restricted to patients with a tumor size < 30 mm as in the STO-3 trial [13]. The IKA trial included both lymph node-negative and lymph-positive patients, and in the IKA post hoc analysis, we selected only ER+HER2− patients, while in the STO-3 trial also, ER+ HER2 + and ER-HER2 − patients were included in the secondary analysis. In addition, our post hoc study is five times smaller than the STO-3 secondary study, resulting in less than ten ultralow-risk patients at risk after 15 years, which makes long-term estimates imprecise. Of note, when focusing on the 10-year and 15-year BCSS data, our data are very similar to the STO-3 data [13].
In the STO-3 trial, 15% of node-negative ER+ HER2 any patients and 19% of the ER+HER2− patients had an ultralow-risk tumor [13], which is very close to the 20% found in our study. Importantly, both trials mainly ran in an era before the introduction of mammography screening, and hence, current incidence of node-negative, ER+HER2− ultralow-risk tumors among postmenopausal women may even be higher, as earlier observed for 70-gene signature low-risk tumors [26].
The recursive partitioning survival tree [13] analyses of our study and the STO-3 study may help guide postmenopausal, node-negative, HER2-negative patients who experience a substantial deterioration in the quality of life while taking adjuvant endocrine therapy. In the case of an ultralow-risk tumor smaller than or equal to 30 mm in size or a low-risk tumor less than or equal to 20 mm in size, abandoning adjuvant endocrine therapy early will only have a marginal impact on long-term breast cancer-specific survival. On the other hand, for patients with a low-risk but no ultralow-risk tumor over 20 mm in size, or for patients with a high-risk tumor, the substantial long-term recurrence risk may motivate these patients to adhere to their endocrine therapy. Repeatedly, studies have shown that around 10–30% of patients stop adjuvant endocrine therapy early or do not start at all [7]. This also implicates that the true benefit of adjuvant endocrine therapy is larger than observed in meta-analyses of randomized trials that generally use the intention-to-treat principle [7, 27].
In addition, currently, aromatase inhibitors have predominantly replaced tamoxifen as adjuvant endocrine therapy in the postmenopausal setting, leading to a further reduction in recurrence risk [1]. Since low MAI (< 8/2 mm2) has recently been reported to be a useful biomarker for endocrine therapy sensitivity [15], and in our study, 87% of ultralow-risk tumors had a low MAI, while only 30% of high-risk tumors had a low MAI, it is highly likely that already 1–3 years of adjuvant endocrine therapy will substantially reduce the already limited risk of breast cancer recurrence in ultralow-risk, node-negative, postmenopausal patients. In the same vein, patients with ER+ HER− ultralow-risk tumors are enriched for PR positivity. While PR positivity has been associated with tamoxifen sensitivity, the absolute breast cancer recurrence risk for ultralow-risk patients is already very low, resulting in a very small absolute benefit from tamoxifen treatment.
Besides 70-gene signature, several other multigene prognostic tests have been developed, and some of these have entered daily clinical practice [28]. Most evidence has been compiled for these tests to be used for the decision to forego adjuvant chemotherapy in node-negative, ER+HER2− postmenopausal patients (e.g., Oncotype DX, MammaPrint, Endopredict, Prosigna, and Breast Cancer Index). Most secondary analyses of large randomized clinical trials, such as ATAC, ABCSG6, ABCSG8, and STO-3 have been instrumental in demonstrating clinical utility [29,30,31,32,33]. Only for MammaPrint, 70-gene signature and Oncotype DX have level I evidence been generated with prospective, randomized clinical trials that also included premenopausal women [11, 34]. Secondary analyses of the STO-3 trial have indicated that the70-gene signature and Breast Cancer Index multigene tests may help in selecting node-negative, postmenopausal ER+ HER- breast cancer patients for whom limited or no adjuvant tamoxifen would suffice [13, 33]. Data presented here further support the MammaPrint 70-gene signature for this indication. Moreover, a prospective trial to further validate this finding seems unrealistic in this group of patients, due to the low event rate and the long follow-up that will be needed.
In patients with ER+HER2− node-positive breast cancer, the 70-gene signature predicts BCSS difference between genomic high risk and low risk (including the ultralow-risk), but not between ultralow-risk and low-risk patients. In an exploratory analysis, the second variable predicting BCSS in this group of patients is tumor size (below or above 20 mm), resulting in no BCSS event out of seven patients in the 70-gene low risk (including ultralow) and tumor size < 20 mm group. Although the median follow-up in this group is short (5 years) and the numbers are small, there is a significant survival difference between the groups. It would be very interesting to see if this result can be confirmed in other series with node-positive patients treated without chemotherapy and only limited endocrine therapy.
Limitations of this study include the small sample size. Only 736 of the 1662 (44%) patients were subtyped and for 72% (346/482) of the ER+HER2− patients sufficient left-over material was available. Furthermore, due to the use of old FFPE material, 61% (211/346) of the samples failed the quality criteria of the 70-gene signature. There were no differences between patients with tumors that resulted in a 70-gene signature score and the remaining ER+HER2− patients, reassuring that there was no obvious selection bias. Furthermore, at the time of the study, tamoxifen was used as standard of care, while nowadays, treatment with an aromatase inhibitor (AI) is more common in postmenopausal patients. However, it is expected that ultralow-risk patients with no or only limited tamoxifen treatment would also have an excellent prognosis with no or limited AI treatment. Finally, we lacked information on tamoxifen treatment non-compliance that generally ranges between 10 and 30% [7]. However, non-compliance would result in underestimating outcome, meaning that with 100% compliance, tamoxifen-treated patients would even have a better outcome than reported here.
Strengths of this study include the uniqueness of well-annotated data of patients participating in a trial randomized between no treatment or only a short duration of adjuvant endocrine therapy and with 20 years of follow-up data available.
In conclusion, an ultralow-risk MammaPrint 70-gene signature test result can help avoid systemic overtreatment in postmenopausal patients with node-negative, estrogen receptor-positive, HER2-negative breast cancer and has the potential to improve shared decision making by clinicians and patients regarding limited or no adjuvant endocrine therapy.
Data availability
The data that support the findings of this study can be found in supplement file S3. Other data are available from the authors upon reasonable request and with permission of The Netherlands Institute.
References
Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA, Giordano SH, Hudis CA, Solky AJ, Stearns V et al (2019) Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol 37(5):423–438
Early Breast Cancer Trialists’ Collaborative Group (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386(101):1341–1352
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC et al (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–784
Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A, Bonfill X et al (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381(9869):805–816
Paranjpe R, John G, Trivedi M, Abughosh S (2019) Identifying adherence barriers to oral endocrine therapy among breast cancer survivors. Breast Cancer Res Treat 174(2):297–305
Pistilli B, Paci A, Ferreira AR, Meglio AD, Poinsignon V, Bardet A, Menvielle G, Dumas A, Pinto S, Dauchy S et al (2020) Serum detection of nonadherence to adjuvant tamoxifen and breast cancer recurrence risk. J Clin Oncol 38(24):2762–2772
Early Breast Cancer Trialists’ Collaborative Group, Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C et al (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–784
Goldvaser H, Barnes TA, Seruga B, Cescon DW, Ocana A, Ribnikar D, Amir E (2018) Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst 110(1):31–39
Knauer M, Mook S, Rutgers EJ, Bender RA, Hauptmann M, van de Vijver MJ, Koornstra RH, Bueno-de-Mesquita JM, Linn SC, van’t Veer LJ (2010) The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res Treat 120(3):655–661
Bueno-de-Mesquita JM, van Harten WH, Retel VP, van’t Veer LJ, van Dam FS, Karsenberg K, Douma KF, van Tinteren H, Peterse JL, Wesseling J et al (2007) Use of 70-gene signature to predict prognosis of patients with node-negative breast cancer: a prospective community-based feasibility study (RASTER). Lancet Oncol 8(12):1079–1087
Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga J-Y, Brain E, Causeret S, DeLorenzi M et al (2016) 70-gene signature as an aid to treatment decisions in early-stage breast cancer. New England Journal of Medicine 375(8):717–729
Delahaye LJMJ, Drukker CA, Dreezen C, Witteveen A, Chan B, Snel M, Beumer IJ, Bernards R, Audeh MW, van’t Veer LJ et al (2017) A breast cancer gene signature for indolent disease. Breast Cancer Res Treat 164(2):461–466
Esserman LJ, Yau C, Thompson CK, van’t Veer LJ, Borowsky AD, Hoadley KA, Tobin NP, Nordenskjold B, Fornander T, Stal O et al (2017) Use of molecular tools to identify patients with indolent breast cancers with ultralow risk over 2 decades. JAMA Oncol 3(11):1503–1510
Vermorken JBBJ, Taat CW, van de Slee PHT, Hennipman A, Norman JWR, Rozendaal KJ, van Tinteren H, Huldij J, Benraadt J (1998) Adjuvant tamoxifen in breast cancer: interim results of a comprehensive cancer center Amsterdam trial. Breast Cancer Res Treat 50(329):283
Beelen K, Opdam M, Severson T, Koornstra R, Vincent A, Wesseling J, Sanders J, Vermorken J, van Diest P, Linn S (2018) Mitotic count can predict tamoxifen benefit in postmenopausal breast cancer patients while Ki67 score cannot. BMC Cancer 18(1):761
Sauerbrei W, Taube SE, McShane LM, Cavenagh MM, Altman DG (2018) Reporting recommendations for tumor marker prognostic studies (REMARK): an abridged explanation and elaboration. J Natl Cancer Inst 110(8):803–811
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717
Beelen K, Opdam M, Severson TM, Koornstra RH, Vincent AD, Wesseling J, Muris JJ, Berns EM, Vermorken JB, van Diest PJ et al (2014) Phosphorylated p-70S6K predicts tamoxifen resistance in postmenopausal breast cancer patients randomized between adjuvant tamoxifen versus no systemic treatment. Breast Cancer Res 16(1):R6
Glas AM, Floore A, Delahaye LJ, Witteveen AT, Pover RC, Bakx N, Lahti-Domenici JS, Bruinsma TJ, Warmoes MO, Bernards R et al (2006) Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics 7:278
Krijgsman O, Roepman P, Zwart W, Carroll JS, Tian S, de Snoo FA, Bender RA, Bernards R, Glas AM (2012) A diagnostic gene profile for molecular subtyping of breast cancer associated with treatment response. Breast Cancer Res Treat 133(1):37–47
Drukker CA, van Tinteren H, Schmidt MK, Rutgers EJ, Bernards R, van de Vijver MJ, Van’t Veer LJ (2014) Long-term impact of the 70-gene signature on breast cancer outcome. Breast Cancer Res Treat 143(3):587–592
van’t Veer LJ, Yau C, Yu NY, Benz CC, Nordenskjöld B, Fornander T, Stål O, Esserman LJ, Lindström LS (2017) Tamoxifen therapy benefit for patients with 70-gene signature high and low risk. Breast Cancer Res Treat 166(2):593–601
Gourgou-Bourgade S, Cameron D, Poortmans P, Asselain B, Azria D, Cardoso F, A’Hern R, Bliss J, Bogaerts J, Bonnefoi H et al (2015) Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials). Ann Oncol 26(5):873–879
Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17(4):343–346
Cardozo JL, Drukker C, Schmidt M, Veer LVT, Glas A, Witteveen A, Cardoso F, Piccart-Gebhart MJ, Poncet C, Rutgers EJ (2021) Outcome of patients with an ultralow risk 70-gene signature in the MINDACT trial. J Clin Oncol 39(15_suppl):500–500
Esserman LJ, Shieh Y, Rutgers EJ, Knauer M, Retel VP, Mook S, Glas AM, Moore DH, Linn S, van Leeuwen FE et al (2011) Impact of mammographic screening on the detection of good and poor prognosis breast cancers. Breast Cancer Res Treat 130(3):725–734
Chia SK, Wolff AC (2011) With maturity comes confidence: EBCTCG tamoxifen update. Lancet 378(9793):747–749
Kwa M, Makris A, Esteva FJ (2017) Clinical utility of gene-expression signatures in early stage breast cancer. Nat Rev Clin Oncol 14(10):595–610
Sestak I, Buus R, Cuzick J, Dubsky P, Kronenwett R, Denkert C, Ferree S, Sgroi D, Schnabel C, Baehner FL et al (2018) Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol 4(4):545–553
Dubsky P, Brase JC, Jakesz R, Rudas M, Singer CF, Greil R, Dietze O, Luisser I, Klug E, Sedivy R et al (2013) The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2− breast cancer patients. Br J Cancer 109(12):2959–2964
Gnant M, Filipits M, Greil R, Stoeger H, Rudas M, Bago-Horvath Z, Mlineritsch B, Kwasny W, Knauer M, Singer C et al (2014) Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol 25(2):339–345
Filipits M, Dubsky P, Rudas M, Greil R, Balic M, Bago-Horvath Z, Singer CF, Hlauschek D, Brown K, Bernhisel R et al (2019) Prediction of distant recurrence using endopredict among women with ER(+), HER2(−) node-positive and node-negative breast cancer treated with endocrine therapy only. Clin Cancer Res 25(13):3865–3872
Jerevall PL, Ma XJ, Li H, Salunga R, Kesty NC, Erlander MG, Sgroi DC, Holmlund B, Skoog L, Fornander T et al (2011) Prognostic utility of HOXB13:IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br J Cancer 104(11):1762–1769
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr et al (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379(2):111–121
Acknowledgements
We would like to acknowledge the Core Facility Molecular Pathology & Biobanking (CFMPB) of the Netherlands Cancer Institute for supplying tissue material and lab support. We would also like to thank all patients, participating hospitals and involved general practitioners.
Funding
This work was supported by Grants from Agendia®, and A Sister’s Hope. A Sister’s Hope did not have any influence on study design; data collection; and/or project management; data analysis, interpretation; or manuscript preparation, review, or approval. Agendia® generated MammaPrint® results for all submitted tumor samples, blinded to clinical outcome data. Agendia® did not have any influence on study design, or data analysis. Two employees from Agendia® were involved in project management, data interpretation, manuscript review, and approval (MK, AG).
Author information
Authors and Affiliations
Contributions
SCL conceived the study. The study was designed by MO, VvdN, HvT, and SCL. Data were contributed by MO, MK, AG, HvT, JBV, and PvD. All data were analyzed by MO, VvdN and interpreted by all authors. MO, FSH, and SCL drafted the manuscript. All authors listed, critically reviewed, and approved the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interest
SCL reports grants from Agendia® and A Sister's Hope during the conduct of the study. SCL is an advisory board member for Cergentis, and IBM, and received institutional research support from AstraZeneca, Eurocept-pharmaceuticals, Genentech, Novartis, Pfizer, Roche, Tesaro, and Immunomedics. In addition, SCL received institutional non-financial support from AstraZeneca, Genentech, Novartis, Roche, Tesaro, and Immunomedics and institutional educational financial support from Bayer and Daiichi-Sankyo outside of this study. MK: Full/Part-time employment: Agendia. AG: Full/Part-time employment: Agendia. JBV reports an advisory/consultancy role for Innate Pharma, Merck Sharp & Dome Corp, PCI Biotech, Nanobiotix, WntResearch, and having received a Speaker Bureau/Expert testimony, lecture fee from Merck-Serono, and MSD. All other authors have declared no conflicts of interest.
Ethical approval
The IKA trial was approved by the central ethics committee of the Netherlands Cancer Institute. The trial was performed in accordance with the Declaration of Helsinki. For this retrospective translational study, no additional consent was required according to Dutch legislation, since the use of archival pathology left-over material does not interfere with patient care. This was also discussed with the Ethical, Legal, Social Implications (ELSI) service desk of the Netherlands. Tumor tissue was handled according to the Dutch code of conduct for responsible use of human tissue in the context of health research.
Consent to participate
All participating patients gave informed consent before randomization and agreed to be included in future survival analysis. For this retrospective translational study, no additional consent was required according to Dutch legislation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10549_2022_6618_MOESM1_ESM.pdf
Supplementary file1 Online Resource 1. Kaplan-Meier Plots of Breast Cancer-Specific Survival in node-negative and node-positive patients without censoring second tumor. Breast cancer-specific survival of the patients stratified based on 70-gene MammaPrint risk score for (A) node-negative patients and (B) node-positive patients. (PDF 148 kb)
10549_2022_6618_MOESM2_ESM.docx
Supplementary file2 Online Resource 2. Table with survival rates by 70-gene signature risk classification with 95% confidence intervals for Breast Cancer-Specific Survival at 10, 15 and 20 years split by nodal status without censoring for second tumor. (DOCX 12 kb)
10549_2022_6618_MOESM3_ESM.csv
Supplementary file3 Online Resource 3. Data file of all 135 patients with 70-gene signature, clinicopathological characteristics and survival data. (CSV 22 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Opdam, M., van der Noort, V., Kleijn, M. et al. Limiting systemic endocrine overtreatment in postmenopausal breast cancer patients with an ultralow classification of the 70-gene signature. Breast Cancer Res Treat 194, 265–278 (2022). https://doi.org/10.1007/s10549-022-06618-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06618-z