Abstract
Purpose
Hypoxia-Inducible Factor HIF1α and lactate dehydrogenase LDHA drive anaerobic tumor metabolism and define clinical aggressiveness. We investigated their expression in breast cancer and their role in immune response and prognosis of breast cancer.
Methods
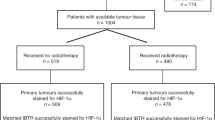
Tissue material from 175 breast cancer patients treated in a prospective study were analyzed with immunohistochemistry for HIF1α and LDH5 expression, in parallel with the tumor-infiltrating lymphocyte TIL-density and tertiary lymphoid structure TLS-density.
Results
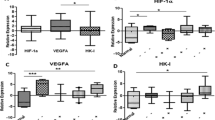
High LDH5 expression was noted in 48/175 tumors, and this was related to HIF1α overexpression (p < 0.0001), triple-negative TNBC histology (p = 0.01), poor disease-specific survival (p < 0.007), metastasis (p < 0.01), and locoregional recurrence (p = 0.03). High HIF1α expression, noted in 39/175 cases, was linked with low steroid receptor expression (p < 0.05), her2 overexpression (p = 0.01), poor survival (p < 0.04), and high metastasis rates (p < 0.004). High TIL-density in the invading tumor front (TILinv) was linked with low LDH5 and HIF expression (p < 0.0001) and better prognosis (p < 0.02). High TIL-density in inner tumor areas (TILinn) was significantly linked with TNBC. Multivariate analysis showed that PgR-status (p = 0.003, HR 2.99, 95% CI 1.4–6.0), TILinv (p = 0.02, HR 2.31, 95% CI 1.1–4.8), LDH5 (p = 0.01, HR 2.43, 95% CI 1.2–5.0), N-stage (p = 0.04, HR 2.42, 95% CI 1.0–5.8), T-stage (p = 0.04, HR 2.31, 95% CI 1.0–5.1), and her2 status (p = 0.05, HR 2.01, 95% CI 1.0–4.2) were independent variables defining death events.
Conclusion
Overexpression of LDH5, an event directly related to HIF1α overexpression, characterizes a third of breast tumors, which is more frequent in TNBC. Both HIF1α and LDH5 define cold breast cancer microenvironment and poor prognosis. A rational is provided to study further whether metabolic manipulations targeting HIF and LDH5 may enhance the antitumor immune response in breast cancer.





Similar content being viewed by others
Data availability
All tissue samples and patient data are stored in the archives of the Department of Pathology and Department of Radiotherapy and Oncology of the Democritus University of Thrace, and can become available upon reasonable request.
Abbreviations
- HIF1α¨:
-
Hypoxia-Inducible Factor 1α
- LDHA:
-
Lactate dehydrogenase A
- TNBC:
-
Triple negative breast cancer
- TIL:
-
Tumor-infiltrating lymphocytes
- inv:
-
Invading tumor front; inn: inner tumor areas
- TLS:
-
Tertiary lymphoid structure
- PD-L1:
-
Programmed death-ligand 1
- PD-1:
-
Programmed cell death protein 1
- ER:
-
Estrogen receptor
- PgR:
-
Progesterone receptor
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386. https://doi.org/10.1002/ijc.29210
Ali HR, Glont SE, Blows FM, Provenzano E, Dawson SJ, Liu B, Hiller L, Dunn J, Poole CJ, Bowden S, Earl HM, Pharoah PD, Caldas C (2015) PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol 26:1488–1493. https://doi.org/10.1093/annonc/mdv19
Dias AS, Almeida CR, Helguero LA, Duarte IF (2019) Metabolic crosstalk in the breast cancer microenvironment. Eur J Cancer 121:154–171. https://doi.org/10.1016/j.ejca.2019.09.002
Naik A, Decock J (2020) Lactate metabolism and immune modulation in breast cancer: a focused review on triple-negative breast tumors. Front Oncol 10:598626. https://doi.org/10.3389/fonc.2020.598626
Semenza GL (2013) HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 123:3664–3671. https://doi.org/10.1172/JCI67230
Vaupel P, Schmidberger H, Mayer A (2019) The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol 95(7):912–919. https://doi.org/10.1080/09553002.2019.15896
Johnson JM, Cotzia P, Fratamico R, Mikkilineni L, Chen J, Colombo D, Mollaee M, Whitaker-Menezes D, Domingo-Vidal M, Lin Z, Zhan T, Tuluc M, Palazzo J, Birbe RC, Martinez-Outschoorn UE (2017) MCT1 in invasive ductal carcinoma: monocarboxylate metabolism and aggressive breast cancer. Front Cell Dev Biol 5:27. https://doi.org/10.3389/fcell.2017.00027
Cheung SM, Husain E, Masannat Y, Miller ID, Wahle K, Heys SD, He J (2020) Lactate concentration in breast cancer using advanced magnetic resonance spectroscopy. Br J Cancer 123:261–267. https://doi.org/10.1038/s41416-020-0886-7
Damgaci S, Ibrahim-Hashim A, Enriquez-Navas PM, Pilon-Thomas S, Guvenis A, Gillies RJ (2018) Hypoxia and acidosis: immune suppressors and therapeutic targets. Immunology 154:354–362. https://doi.org/10.1111/imm.12917
Koukourakis IM, Panteliadou M, Giakzidis AG, Nanos C, Abatzoglou I, Giatromanolaki A, Koukourakis MI (2021) Long-term results of postoperative hypofractionated accelerated breast and lymph node radiotherapy (HypoAR) with hypofractionated boost. Curr Oncol 28(5):3474–3487. https://doi.org/10.3390/curroncol28050300
Koukourakis MI, Tsoutsou PG, Abatzoglou IM, Sismanidou K, Giatromanolaki A, Sivridis E (2009) Hypofractionated and accelerated radiotherapy with subcutaneous amifostine cytoprotection as short adjuvant regimen after breast-conserving surgery: interim report. Int J Radiat Oncol Biol Phys 74(4):1173–1180. https://doi.org/10.1016/j.ijrobp.2008.09.016
Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, Harris AL, Tumour and Angiogenesis Research Group (2003) Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer 89(5):877–885. https://doi.org/10.1038/sj.bjc.6601205
Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL (2000) The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 157:411–421. https://doi.org/10.1016/s0002-9440(10)64554-3
Giatromanolaki A, Harris AL, Koukourakis MI (2021) The prognostic and therapeutic implications of distinct patterns of argininosuccinate synthase 1 (ASS1) and arginase-2 (ARG2) expression by cancer cells and tumor stroma in non-small-cell lung cancer. Cancer Metab 9:28. https://doi.org/10.1186/s40170-021-00264-7
Koukourakis MI, Bentzen SM, Giatromanolaki A, Wilson GD, Daley FM, Saunders MI, Dische S, Sivridis E, Harris AL (2006) Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol 24(5):727–735. https://doi.org/10.1200/JCO.2005.02.7474
Masoud GN, Li W (2015) HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 5:378–389. https://doi.org/10.1016/j.apsb.2015.05.007
Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ, van der Wall E (2003) Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node-negative breast carcinoma. Cancer 97:1573–1581. https://doi.org/10.1002/cncr.11246
Giatromanolaki A, Koukourakis MI, Simopoulos C, Polychronidis A, Gatter KC, Harris AL, Sivridis E (2004) c-erbB-2 related aggressiveness in breast cancer is hypoxia-inducible factor-1alpha dependent. Clin Cancer Res 10:7972–7977. https://doi.org/10.1158/1078-0432.CCR-04-1068
Wang W, He YF, Sun QK, Wang Y, Han XH, Peng DF, Yao YW, Ji CS, Hu B (2014) Hypoxia-inducible factor 1α in breast cancer prognosis. Clin Chim Acta 428:32–37. https://doi.org/10.1016/j.cca.2013.10.018
Wyss CB, Duffey N, Peyvandi S, Barras D, Martinez Usatorre A, Coquoz O, Romero P, Delorenzi M, Lorusso G, Rüegg C (2021) Gain of HIF1 activity and Loss of miRNA let-7d promote breast cancer metastasis to the brain via the PDGF/PDGFR axis. Cancer Res 81:594–605. https://doi.org/10.1158/0008-5472.CAN-19-3560
Semenza GL, Jiang BH, Leung SW, Passantino R, Concordat JP, Maire P, Giallongo A (1996) Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271:32529–32537. https://doi.org/10.1074/jbc.271.51.32529
Koukourakis MI, Giatromanolaki A (2019) Warburg effect, lactate dehydrogenase, and radio/chemotherapy efficacy. Int J Radiat Biol 95:408–426. https://doi.org/10.1080/09553002.2018
Brown JE, Cook RJ, Lipton A, Coleman RE (2012) Serum lactate dehydrogenase is prognostic for survival in patients with bone metastases from breast cancer: a retrospective analysis in bisphosphonate-treated patients. Clin Cancer Res 18:6348–6355. https://doi.org/10.1158/1078-0432.CCR-12-1397
Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70. https://doi.org/10.1038/nature11412
Dang CV, Le A, Gao P (2009) MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 15(21):6479–6483. https://doi.org/10.1158/1078-0432.CCR-09-0889
Liu X, Meng QH, Ye Y, Hildebrandt MA, Gu J, Wu X (2015) Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with non-metastatic breast cancer. Carcinogenesis 36:243–248. https://doi.org/10.1093/carcin/bgu247
Wang S, Ma L, Wang Z, He H, Chen H, Duan Z, Li Y, Si Q, Chuang TH, Chen C, Luo Y (2021) Lactate dehydrogenase-A (LDH-A) preserves cancer stemness and recruitment of tumor-associated macrophages to promote breast cancer progression. Front Oncol 11:654452. https://doi.org/10.3389/fonc.2021.654452
Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, Renner K, Timischl B, Mackensen A, Kunz-Schughart L, Andreesen R, Krause SW, Kreutz M (2007) Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109:3812–3819. https://doi.org/10.1182/blood-2006-07-035972
Cascone T, McKenzie JA, Mbofung RM, Punt S, Wang Z, Xu C, Williams LJ, Wang Z, Bristow CA, Carugo A, Peoples MD, Li L, Karpinets T, Huang L, Malu S, Creasy C, Leahey SE, Chen J, Chen Y, Pelicano H, Bernatchez C, Gopal YNV, Heffernan TP, Hu J, Wang J, Amaria RN, Garraway LA, Huang P, Yang P, Wistuba II, Woodman SE, Roszik J, Davis RE, Davies MA, Heymach JV, Hwu P, Peng W (2018) Increased Tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab 27:977-987.e4. https://doi.org/10.1016/j.cmet.2018.02.02499
Haas R, Smith J, Rocher-Ros V, Nadkarni S, Montero-Melendez T, D’Acquisto F, Bland EJ, Bombardieri M, Pitzalis C, Perretti M, Marelli-Berg FM, Mauro C (2015) Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol 13:e1002202. https://doi.org/10.1371/journal.pbio.1002202
Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, Wang Z, Quinn WJ 3rd, Kopinski PK, Wang L, Akimova T, Liu Y, Bhatti TR, Han R, Laskin BL, Baur JA, Blair IA, Wallace DC, Hancock WW, Beier UH (2017) Foxp3 reprograms T cell metabolism to function in low-glucose. High-Lactate Environ Cell Metab 25:1282-1293.e7. https://doi.org/10.1016/j.cmet.2016.12.018
Li N, Kang Y, Wang L, Huff S, Tang R, Hui H, Agrawal K, Gonzalez GM, Wang Y, Patel SP, Rana TM (2020) ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in the tumor microenvironment. Proc Natl Acad Sci U S A 117:20159–20170. https://doi.org/10.1073/pnas.1918986117
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, Schmitt WD, Blohmer JU, Karn T, Pfitzner BM, Kümmel S, Engels K, Schneeweiss A, Hartmann A, Noske A, Fasching PA, Jackisch C, van Mackelenbergh M, Sinn P, Schem C, Hanusch C, Untch M, Loibl S (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19:40–50. https://doi.org/10.1016/S1470-2045(17)30904-X
Zhang NN, Qu FJ, Liu H, Li ZJ, Zhang YC, Han X, Zhu ZY, Lv Y (2021) Prognostic impact of tertiary lymphoid structures in breast cancer prognosis: a systematic review and meta-analysis. Cancer Cell Int 21:536. https://doi.org/10.1186/s12935-021-02242-x
Miligy I, Mohan P, Gaber A, Aleskandarany MA, Nolan CC, Diez-Rodriguez M, Mukherjee A, Chapman C, Ellis IO, Green AR, Rakha EA (2017) Prognostic significance of tumour infiltrating B lymphocytes in breast ductal carcinoma in situ. Histopathology 71(2):258–268. https://doi.org/10.1111/his.13217
Qian F, Qingping Y, Linquan W, Xiaojin H, Rongshou W, Shanshan R, Wenjun L, Yong H, Enliang L (2017) High tumor-infiltrating FoxP3+ T cells predict poor survival in estrogen receptor-positive breast cancer: a meta-analysis. Eur J Surg Oncol 43(7):1258–1264. https://doi.org/10.1016/j.ejso.2017.01.011
Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, Kastenberger M, Bogdan C, Schleicher U, Mackensen A, Ullrich E, Fichtner-Feigl S, Kesselring R, Mack M, Ritter U, Schmid M, Blank C, Dettmer K, Oefner PJ, Hoffmann P, Walenta S, Geissler EK, Pouyssegur J, Villunger A, Steven A, Seliger B, Schreml S, Haferkamp S, Kohl E, Karrer S, Berneburg M, Herr W, Mueller-Klieser W, Renner K, Kreutz M (2016) LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab 24:657–671. https://doi.org/10.1016/j.cmet.2016.08.011
Li W, Tanikawa T, Kryczek I, Xia H, Li G, Wu K, Wei S, Zhao L, Vatan L, Wen B, Shu P, Sun D, Kleer C, Wicha M, Sabel M, Tao K, Wang G, Zou W (2018) Aerobic glycolysis controls myeloid-derived suppressor cells and tumor immunity via a specific CEBPB Isoform in triple-negative breast cancer. Cell Metab 28:87-103.e6. https://doi.org/10.1016/j.cmet.2018.04.022
Funding
The study has been supported by the Special Account of the Democritus University of Thrace, number 8106. S.P. received a grant support from the IKY foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by AG, MK, EB, and SK. Immunohistochemistry was performed by AGG and SP. The first draft of the manuscript was written by MK and AG, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University Hospital of Alexandroupolis (Date.26-11-2018/No11).
Consent to participate
Written informed consent was obtained from the parents.
Consent to publish
The authors affirm that human research participants provided informed consent for anonymous analysis and publication of their clinical and laboratory data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giatromanolaki, A., Gkegka, A.G., Pouliliou, S. et al. Hypoxia and anaerobic metabolism relate with immunologically cold breast cancer and poor prognosis. Breast Cancer Res Treat 194, 13–23 (2022). https://doi.org/10.1007/s10549-022-06609-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06609-0