Abstract
Background
The management of high-risk breast lesions diagnosed on image-guided core biopsy remains controversial. We implemented a high-risk breast conference attended by breast pathologists, imagers, and surgeons to prospectively review all contemporary cases in order to provide a consensus recommendation to either surgically excise or follow on imaging at 6-month intervals for a minimum of 2 years.
Methods
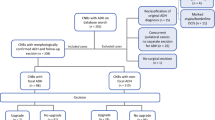
Between May, 2015 and June, 2019, 127 high-risk lesions were discussed. Of these 127 cases, 116 had concordant radiology–pathology (rad–path) findings. The remaining 11 patients had discordant rad–path findings. Of the 116 concordant cases, 6 were excluded due to lack of the first imaging follow-up until analysis. Of the remaining 110 patients, 43 had atypical ductal hyperplasia (ADH), 12 had lobular carcinoma in situ (LCIS), 19 had atypical lobular hyperplasia (ALH), 33 had radial scar (RS), 2 had flat epithelial atypia (FEA), and 1 had mucocele-like lesion (ML). We recommended excision for ADH if there were > 2 ADH foci or < 90% of the associated calcifications were removed. For patients with LCIS or ALH, we recommended excision if the LCIS or ALH was associated with microcalcifications or the LCIS was extensive. We recommended excision of RS when < 1/2 of the lesion was biopsied. We recommended all patients with FEA and ML for 6-month follow-up.
Results
Following conference-derived consensus for excision, of the 27 ADH excised, 9 were upgraded to invasive carcinoma or ductal carcinoma in situ. Of the six LCIS cases recommended for excision, none were upgraded. Nine excised radial scars revealed no upgrades. Additionally, 3 patients with ADH, 2 with ALH, 1 with LCIS, and 2 with RS underwent voluntary excision, and none were upgraded. All other patients (13 with ADH, 5 LCIS, 17 ALH, 22 RS, 2 FEA and 1 ML) were followed with imaging, and none revealed evidence of disease progression during follow-up (187–1389 days). All 11 rad–path discordant cases were excised with 2 upgraded to carcinoma.
Conclusions
The results of this prospective study indicate that high-risk breast lesions can be successfully triaged to surgery versus observation following establishment of predefined firm guidelines and performance of rigorous rad–path correlation.

Similar content being viewed by others
References
Dahabreh IJ, Wieland LS, Adam GP et al (2014) AHRQ comparative effectiveness reviews: Core needle and open surgical biopsy for diagnosis of breast lesions: an update to the 2009 report. Rockville (MD), Agency for Healthcare Research and Quality (US)
Allison KH, Abraham LA, Weaver DL et al (2015) Trends in breast biopsy pathology diagnoses among women undergoing mammography in the United States: a report from the Breast Cancer Surveillance Consortium. Cancer 121:1369–1378
Menes TS, Rosenberg R, Balch S et al (2014) Upgrade of high-risk breast lesions detected on mammography in the Breast Cancer Surveillance Consortium. Am J Surg 207:24–31
Foster MC, Helvie MA, Gregory NE et al (2004) Lobular carcinoma in situ or atypical lobular hyperplasia at core-needle biopsy: is excisional biopsy necessary? Radiology 231:813–819
Sneige N, Lim SC, Whitman GJ et al (2003) Atypical ductal hyperplasia diagnosis by directional vacuum-assisted stereotactic biopsy of breast microcalcifications. Considerations for surgical excision. Am J Clin Pathol 119:248–253
Eby PR, Ochsner JE, DeMartini WB et al (2008) Is surgical excision necessary for focal atypical ductal hyperplasia found at stereotactic vacuum-assisted breast biopsy? Ann Surg Oncol 15:3232–3238
Li X, Aho M, Newell MS et al (2020) Papilloma diagnosed on core biopsies has a low upgrade rate. Clin Imaging 60:67–74
Ma Z, Arciero CA, Styblo TM et al (2020) Patients with benign papilloma diagnosed on core biopsies and concordant pathology–radiology findings can be followed: experiences from multi-specialty high-risk breast lesion conferences in an academic center. Breast Cancer Res Treat 183:577–584
Page DL, Dupont WD, Rogers LW et al (1985) Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer 55:2698–2708
Tavassoli FA, Norris HJ (1990) A comparison of the results of long-term follow-up for atypical intraductal hyperplasia and intraductal hyperplasia of the breast. Cancer 65:518–529
Page DL, Kidd TE Jr, Dupont WD et al (1991) Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol 22:1232–1239
Guray M, Sahin AA (2006) Benign breast diseases: classification, diagnosis, and management. Oncologist 11:435–449
Moseley TW, Shah SS, Nguyen CV et al (2019) Clinical management of mucocele-like lesions of the breast with limited or no epithelial atypia on core biopsy: experience from two institutions. Ann Surg Oncol 26:3478–3488
Krishnamurthy S, Bevers T, Kuerer H et al (2012) Multidisciplinary considerations in the management of high-risk breast lesions. AJR Am J Roentgenol 198:W132–W140
Sewell CW (2004) Pathology of high-risk breast lesions and ductal carcinoma in situ. Radiol Clin N Am 42:821–830
Dupont WD, Page DL (1985) Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 312:146–151
Hartmann LC, Sellers TA, Frost MH et al (2005) Benign breast disease and the risk of breast cancer. N Engl J Med 353:229–237
London SJ, Connolly JL, Schnitt SJ et al (1992) A prospective study of benign breast disease and the risk of breast cancer. JAMA 267:941–944
Neal L, Sandhu NP, Hieken TJ et al (2014) Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc 89:536–547
Schmitt FC, Leal C, Lopes C (1995) p53 protein expression and nuclear DNA content in breast intraductal proliferations. J Pathol 176:233–241
Viacava P, Naccarato AG, Bevilacqua G (1999) Different proliferative patterns characterize different preinvasive breast lesions. J Pathol 188:245–251
O’Connell P, Pekkel V, Fuqua SA et al (1998) Analysis of loss of heterozygosity in 399 premalignant breast lesions at 15 genetic loci. J Natl Cancer Inst 90:697–703
Kader T, Hill P, Rakha EA et al (2018) Atypical ductal hyperplasia: update on diagnosis, management, and molecular landscape. Breast Cancer Res 20:39
Danforth DN (2018) Molecular profile of atypical hyperplasia of the breast. Breast Cancer Res Treat 167:9–29
Chen LY, Hu J, Tsang JYS et al (2019) Diagnostic upgrade of atypical ductal hyperplasia of the breast based on evaluation of histopathological features and calcification on core needle biopsy. Histopathology 75:320–328
Kohr JR, Eby PR, Allison KH et al (2010) Risk of upgrade of atypical ductal hyperplasia after stereotactic breast biopsy: effects of number of foci and complete removal of calcifications. Radiology 255:723–730
Mooney KL, Bassett LW, Apple SK (2016) Upgrade rates of high-risk breast lesions diagnosed on core needle biopsy: a single-institution experience and literature review. Mod Pathol 29:1471–1484
Peña A, Shah SS, Fazzio RT et al (2017) Multivariate model to identify women at low risk of cancer upgrade after a core needle biopsy diagnosis of atypical ductal hyperplasia. Breast Cancer Res Treat 164:295–304
Nguyen CV, Albarracin CT, Whitman GJ et al (2011) Atypical ductal hyperplasia in directional vacuum-assisted biopsy of breast microcalcifications: considerations for surgical excision. Ann Surg Oncol 18:752–761
Allison KH, Eby PR, Kohr J et al (2011) Atypical ductal hyperplasia on vacuum-assisted breast biopsy: suspicion for ductal carcinoma in situ can stratify patients at high risk for upgrade. Hum Pathol 42:41–50
Hwang ES, Hyslop T, Lynch T et al (2019) The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: a phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS). BMJ Open 9:e026797
Hwang ES, Malek V (2019) Estimating the magnitude of clinical benefit of local therapy in patients with DCIS. Breast 48(Suppl 1):S34–S38
Nakhlis F, Gilmore L, Gelman R et al (2016) Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in-situ in patients with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol 23:722–728
Murray MP, Luedtke C, Liberman L et al (2013) Classic lobular carcinoma in situ and atypical lobular hyperplasia at percutaneous breast core biopsy: outcomes of prospective excision. Cancer 119:1073–1079
Middleton LP, Sneige N, Coyne R et al (2014) Most lobular carcinoma in situ and atypical lobular hyperplasia diagnosed on core needle biopsy can be managed clinically with radiologic follow-up in a multidisciplinary setting. Cancer Med 3:492–499
Esserman LE, Lamea L, Tanev S et al (2007) Should the extent of lobular neoplasia on core biopsy influence the decision for excision? Breast J 13:55–61
Middleton LP, Grant S, Stephens T et al (2003) Lobular carcinoma in situ diagnosed by core needle biopsy: when should it be excised? Mod Pathol 16:120–129
D’Alfonso TM, Wang K, Chiu YL et al (2013) Pathologic upgrade rates on subsequent excision when lobular carcinoma in situ is the primary diagnosis in the needle core biopsy with special attention to the radiographic target. Arch Pathol Lab Med 137:927–935
Rendi MH, Dintzis SM, Lehman CD et al (2012) Lobular in-situ neoplasia on breast core needle biopsy: imaging indication and pathologic extent can identify which patients require excisional biopsy. Ann Surg Oncol 19:914–921
Calhoun BC (2018) Core needle biopsy of the breast: an evaluation of contemporary data. Surg Pathol Clin 11:1–16
Nakhlis F, Harrison BT, Giess CS et al (2019) Evaluating the rate of upgrade to invasive breast cancer and/or ductal carcinoma in situ following a core biopsy diagnosis of non-classic lobular carcinoma in situ. Ann Surg Oncol 26:55–61
Shamir ER, Chen YY, Chu T et al (2019) Pleomorphic and florid lobular carcinoma in situ variants of the breast: a clinicopathologic study of 85 cases with and without invasive carcinoma from a single academic center. Am J Surg Pathol 43:399–408
Georgian-Smith D, Lawton TJ (2001) Calcifications of lobular carcinoma in situ of the breast: radiologic–pathologic correlation. AJR Am J Roentgenol 176:1255–1259
Brenner RJ, Jackman RJ, Parker SH et al (2002) Percutaneous core needle biopsy of radial scars of the breast: when is excision necessary? AJR Am J Roentgenol 179:1179–1184
Cawson JN, Malara F, Kavanagh A et al (2003) Fourteen-gauge needle core biopsy of mammographically evident radial scars: is excision necessary? Cancer 97:345–351
Resetkova E, Edelweiss M, Albarracin CT et al (2011) Management of radial sclerosing lesions of the breast diagnosed using percutaneous vacuum-assisted core needle biopsy: recommendations for excision based on seven years’ of experience at a single institution. Breast Cancer Res Treat 127:335–343
Matrai C, D’Alfonso TM, Pharmer L et al (2015) Advocating nonsurgical management of patients with small, incidental radial scars at the time of needle core biopsy: a study of 77 cases. Arch Pathol Lab Med 139:1137–1142
Conlon N, D’Arcy C, Kaplan JB et al (2015) Radial scar at image-guided needle biopsy: is excision necessary? Am J Surg Pathol 39:779–785
Andacoglu O, Kanbour-Shakir A, Teh YC et al (2013) Rationale of excisional biopsy after the diagnosis of benign radial scar on core biopsy: a single institutional outcome analysis. Am J Clin Oncol 36:7–11
Linda A, Zuiani C, Furlan A et al (2010) Radial scars without atypia diagnosed at imaging-guided needle biopsy: how often is associated malignancy found at subsequent surgical excision, and do mammography and sonography predict which lesions are malignant? AJR Am J Roentgenol 194:1146–1151
Tang R, Acevedo F, Lanahan C et al (2019) Incidental breast carcinoma: incidence, management, and outcomes in 4804 bilateral reduction mammoplasties. Breast Cancer Res Treat 177:741–748
Ishag MT, Bashinsky DY, Beliaeva IV et al (2003) Pathologic findings in reduction mammaplasty specimens. Am J Clin Pathol 120:377–380
Tadler M, Vlastos G, Pelte MF et al (2014) Breast lesions in reduction mammaplasty specimens: a histopathological pattern in 534 patients. Br J Cancer 110:788–791
Morrow M, Schnitt SJ, Norton L (2015) Current management of lesions associated with an increased risk of breast cancer. Nat Rev Clin Oncol 12:227–238
McCroskey Z, Sneige N, Herman CR et al (2018) Flat epithelial atypia in directional vacuum-assisted biopsy of breast microcalcifications: surgical excision may not be necessary. Mod Pathol 31:1097–1106
Grabenstetter A, Brennan S, Salagean ED et al (2020) Flat epithelial atypia in breast core needle biopsies with radiologic–pathologic concordance: is excision necessary? Am J Surg Pathol 44:182–190
Gibreel WO, Boughey JC (2016) Mucocele-like lesions of the breast: rate of upstaging and cancer development. Ann Surg Oncol 23:3838–3842
Zhang G, Ataya DL, Lebda P et al (2018) Mucocele-like lesions diagnosed on breast core biopsy: low risk of upgrade and subsequent carcinoma. Breast J 24:314–318
Rakha EA, Shaaban AM, Haider SA et al (2013) Outcome of pure mucocele-like lesions diagnosed on breast core biopsy. Histopathology 62:894–898
Edelweiss M, Corben AD, Liberman L et al (2013) Focal extravasatedmucin in breast core needle biopsies: is surgical excision always necessary? Breast J 19:302–309
Funding
Zhongliang Ma is supported by the Science and Technology Project for Colleges of Shandong Province (No. J18KB111).
Author information
Authors and Affiliations
Contributions
Conception and design: XL, ZM, TMS, CAA, HW, MAC. Analysis and interpretation of data: XL, ZM, TMS, CAA, HW, MAC. Manuscript drafting and reviewing: XL, ZM, TMS, CAA, HW, MAC. XL is responsible for the overall content.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, X., Ma, Z., Styblo, T.M. et al. Management of high-risk breast lesions diagnosed on core biopsies and experiences from prospective high-risk breast lesion conferences at an academic institution. Breast Cancer Res Treat 185, 573–581 (2021). https://doi.org/10.1007/s10549-020-05977-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05977-9