Abstract
Purpose
The aim of this study was to compare the rate of chronic adverse effects after a weaker and stronger postoperative analgesia.
Methods
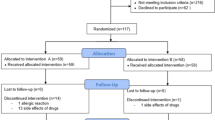
A prospective double-blind randomized study included 117 breast cancer patients receiving tramadol for pain relief for 4 weeks after an axillary lymphadenectomy from 2015 to 2018. Patients with a larger dose received 75/650 mg of tramadol with paracetamol every 8 h and a group with a lower dose received 37.5/325 mg of tramadol with paracetamol every 8 h from the 2nd to the 29th postoperative day. 1 year after surgery, patients were evaluated for the presence of neuropathic pain, chronic pain, arm symptoms and lymphedema.
Results
There was a trend for a lower rate of neuropathic pain after stronger analgesia in comparison to weaker analgesia (p = 0.059). Chronic pain was present in 18% of patients 1 year after the lymphadenectomy. There was no difference in the rate of chronic pain after stronger and weaker postoperative analgesia. Patients had less arm symptoms after a stronger analgesia than after a weaker analgesia (p = 0.02). Furthermore, there was a trend for a lower rate of lymphedema of the forearm after a stronger analgesia than after a lower analgesia (p = 0.078).
Conclusions
The patients who received a stronger postoperative analgesia had less arm symptoms and a better quality of life in comparison to patients who received a weaker analgesia. The patients who received a stronger postoperative analgesia had a statistical trend for less neuropathic pain in comparison to patients who received a weaker analgesia.


Similar content being viewed by others
References
Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Hölzel D (2003) Axilla surgery severely affects quality of life: results of a 5-year prospective study in breast cancer patients. Breast Cancer Res Treat 79:47–57
Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Giuliano AE et al (2007) Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol 25:3657–3663
Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, Kissin M, Mansel RE (2006) Post-operative arm morbidity and quality of life: results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillar treatment in the management of patients with early breast cancer. Breast Cancer Res Treat 95:279–293
Ververs JM, Roumen RM, Vingerhoets AJ, Vreugdenhil G, Coebergh JW, Crommelin MA, Luiten EJ, Repelaer van Driel OJ, Schijven M, Wissing JC et al (2001) Risk, severity and predictors of physical and psychological morbidity after axillary lymph node dissection for breast cancer. Eur J Cancer 37:991–999
Andersen KG, Duriaud HM, Jensen HE, Kroman N, Kehlet H (2015) Predictive factors for the development of persistent pain after breast cancer surgery. Pain 156:2413–2422
Kelly DJ, Ahmad M, Brull SJ (2001) Preemptive analgesia I: physiological pathways and pharmacological modalities. Can J Anaesth 10:1000–1010
Na HS, Oh AY, Koo BW, Lim DJ, Ryu JH, Han JW (2016) Preventive analgesic efficacy of nefopam in acute and chronic pain after breast cancer surgery: a prospective, double-blind, and randomized trial. Medicine (Baltimore) 20:e3705
Strazisar B, Besic N (2013) Comparison of continuous local anaesthetic and systemic pain treatment after axillary lymphadenectomy in breast carcinoma patients: a prospective randomized study. Radiol Oncol 2:145–153
Amr YM, Al-Maksoud YAA (2010) Evaluation of efficacy of the perioperative administration of venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clin J Pain 26:381–385
Reyad RM, Omran AF, Abbas DN, Kamel MA, Shaker EH, Tharwat J, Reyad EM, Hashem T (2019) The possible preventive role of pregabalin in postmastectomy pain syndrome: a double-blinded randomized controlled trial. J Pain Symptom Manag 57:1–9
Kumar S, Goel D, Sharma SK, Ahmad S, Dwivedi P, Deo N, Rani R (2018) A randomised controlled study of the post-operative analgesic efficacy of ultrasound-guided pectoral nerve block in the first 24 h after modified radical mastectomy. Indian J Anaesth 6:436–442
Bravo L, Mico J, Berrocoso E (2017) Discovery and development of tramadol for the treatment of pain. Expert Opin Drug Discov 12:1281–1291
Roussin A, Doazan-d'Ouince O, Géniaux H, Halberer C, French Network of Centre for Evaluation, and Information on Pharmacodependence (Addictovigilance Centres) (2015) Evaluation of abuse and dependence in addiction monitoring systems: tramadol as an example. Therapie 2:203–221
Besic N, Smrekar J, Strazisar B (2020) Severity of acute pain and side effects after weaker and stronger analgesia with tramadol after axillary lymphadenectomy in breast cancer patients: a prospective double-blind randomized study. EJCC (in press)
Wang L, Guyatt GH, Kennedy SA, Romerosa B, Kwon HY, Kaushal A, Chang Y, Craigie S, de Almeida CPB, Couban RJ et al (2016) Predictors of persistent pain after breast cancer surgery: a systematic review and meta-analysis of observational studies. CMAJ 188(14):E352–E361
Larsson IM, Ahm Sørensen J, Bille C (2017) The post-mastectomy pain syndrome-a systematic review of the treatment modalities. Breast J 23:338–343
Waltho D, Rockwell G (2016) Post-breast surgery pain syndrome: establishing a consensus for the definition of post-mastectomy pain syndrome to provide a standardized clinical and research approach: a review of the literature and discussion. Can J Surg 59:342–350
Schou Bredal I, Smeby NA, Ottesen S, Warncke T, Schlichting E (2014) Chronic pain in breast cancer survivors: comparison of psychosocial, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manag 48:852–862
De Oliveira GS, Chang R, Khan SA, Hansen NM, Khan JH, McCarthy RJ, Apkarian AV (2014) Factors associated with the development of chronic pain after surgery for breast cancer: a prospective cohort from a tertiary center in the United States. Breast J 20:9–14
Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H (2012) Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 18:1985–1992
Steyaert A, Forget P, Dubois V, Lavand'homme P, De Kock M (2016) Does the perioperative analgesic/anesthetic regimen influence the prevalence of long-term chronic pain after mastectomy? J Clin Anesth 33:20–25
Steegers MA, Wolters B, Evers AW, Strobbe L, Wilder-Smith OH (2008) Effect of axillary lymph node dissection on prevalence and intensity of chronic and phantom pain after breast cancer surgery. J Pain 9:813–822
Fecho K, Miller NR, Merritt SA, Klauber-DeMore N, Hultman SC, Blau WS (2009) Acute and persistent postoperative pain after breast surgery. Pain Med 10:708–715
Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM (2010) Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 116:5138–5149
Basch E, Iasonos A, McDonough T, Barz A, Culkin A, Kris MG, Scher HI, Schrag D (2006) Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol 7:903–909
Lancet T (2017) The opioid crisis in the USA: a public health emergency. Lancet 390:2016
Jaffe S (2017) Trump administration begins to confront the opioid crisis. Lancet 390:2133–2134
Rose ME (2018) Are prescription opiods driving the opioid crisis? ssumptions vs facts. Pain Med 19:793–807
Petrone D, Kamin M, Olson W (1999) Slowing the titration rate of tramadol HCl reduces the incidence of discontinuation due to nausea and/or vomiting: a double-blind randomized trial. J Clin Pharm Ther 2:115–123
DeLemos B, Richards HM, Vandenbossche J, Ariyawansa J, Natarajan J, Alexander B, Ramakrishna T, Murtaugh T, Stahlberg HJ (2017) Safety, tolerability, and pharmacokinetics of therapeutic and supratherapeutic doses of tramadol hydrochloride in healthy adults: a randomized, double-blind, placebo-controlled multiple-ascending-dose study. Clin Pharmacol Drug Dev 6:592–603
Acknowledgements
The authors thank our research nurses Tjasa Pecnik and Milanka Urankar and research fellow Jerica Novak for their excellent work.
Funding
This study was partly funded by the Ministry of Education, Science and Sport of the Republic of Slovenia, Grant No. P3-0289. This work has been partly funded by an unrestricted research Grant (Trial KCT 04/2015-DORETAonko/si) from Krka, d.d., Novo mesto which also supplied the investigated medicines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N. Besic and B. Strazisar have received a research Grant Trial KCT 04/2015-DORETAonko/si from the pharmaceutical company Krka d.d. Novo mesto. J. Smrekar has received a honorarium for the statistical analysis of our study from the pharmaceutical company Krka d.d. Novo mesto.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study Trial KCT 04/2015-DORETAonko/si, EudraCT Number: 2015-000992-28 met the guidelines of their responsible governmental agency and was reviewed and approved by The National Medical Ethics Committee of the Republic of Slovenia (Approval number 32/03/15). Our study was approved by the Institutional Review Board of the Institute of Oncology Ljubljana.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Besic, N., Smrekar, J. & Strazisar, B. Chronic adverse effects after an axillary lymphadenectomy in breast cancer patients after administering weaker and stronger postoperative analgesia: results of a prospective double-blind randomized study. Breast Cancer Res Treat 182, 655–663 (2020). https://doi.org/10.1007/s10549-020-05713-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05713-3