Abstract
Purpose
The stage-specific survival of young breast cancer patients has improved, likely due to diagnostic and treatment advances. We addressed whether survival improvements have reached all socioeconomic groups in a country with universal health care and national treatment guidelines.
Methods
Using Norwegian registry data, we assessed stage-specific breast cancer survival by education and income level of 7501 patients (2317 localized, 4457 regional, 233 distant and 494 unknown stage) aged 30–48 years at diagnosis during 2000–2015. Using flexible parametric models and national life tables, we compared excess mortality up to 12 years from diagnosis and 5-year relative survival trends, by education and income as measures of socioeconomic status (SES).
Results
Throughout 2000–2015, regional and distant stage 5-year relative survival improved steadily for patients with high education and high income (high SES), but not for patients with low education and low income (low SES). Regional stage 5-year relative survival improved from 85 to 94% for high SES patients (9% change; 95% confidence interval: 6, 13%), but remained at 84% for low SES patients (0% change; − 12, 12%). Distant stage 5-year relative survival improved from 22 to 58% for high SES patients (36% change; 24, 49%), but remained at 11% for low SES patients (0% change; − 19, 19%).
Conclusions
Regional and distant stage breast cancer survival has improved markedly for high SES patients, but there has been little survival gain for low SES patients. Socioeconomic status matters for the stage-specific survival of young breast cancer patients, even with universal health care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stage-specific and overall survival of breast cancer patients has improved over time [1, 2], probably due to advances in diagnostics and treatment. More precise diagnosis of tumor type and stage has enabled treatment to become more tailored to the patient [3,4,5,6]. New treatments have also improved the survival of patients with certain tumor subtypes, for example Herceptin has improved the survival of HER2 positive patients [7, 8]. Although breast cancer survival has been improving, there is concern that patients with low socioeconomic status (SES) have not gained as much from recent advancements [9,10,11,12,13,14].
Like several other countries, Norway has a universal tax funded health care system with the aim to minimize socioeconomic differences in access to diagnostic and treatment care. A nationwide screening program, gradually introduced during 1996–2004, has also ensured universal access to early detection of breast cancer for women aged 50–69 years in Norway. However, younger women may have different diagnostic and care seeking behavior than screen-aged women. Young women fare worse than screen-aged women in terms of breast cancer survival, even after adjustment for tumor characteristics [15,16,17].
Thus, we were interested to know whether access to universal health care has been sufficient to ensure that breast cancer survival has improved for young women from all socioeconomic backgrounds. Studies of socioeconomic inequalities in survival of young patients are lacking. Only a few studies have assessed socioeconomic inequalities in stage-specific survival [18,19,20,21], and none have assessed trends over time.
We took advantage of high-quality Norwegian registry data with individually linked education and income information, to compare trends over time in the stage-specific survival of young women diagnosed before entry to the Breast Cancer Screening Program. We aimed to determine whether survival improvements have reached all socioeconomic groups in a country with universal health care and national treatment guidelines.
Materials and methods
Study design and materials
Using a cohort study design, we assessed the relative survival of all women in Norway diagnosed with invasive breast cancer between Jan 2000 and Dec 2015 at age 30 to 48 years. This age range ensured most patients had completed their education and started earning income, but not yet been invited to mammography screening, before diagnosis. The target screening age in Norway is 50–69 years, although some counties start at 49 years. Breast cancer patients were identified via the nationwide Cancer Registry of Norway, which has had mandatory reporting of new cancer cases since 1953 and is 99% complete [22]. Demographic and socioeconomic characteristics of patients were individually linked from the Central Population Registry, National Education database and Register for Personal Tax Payers.
Study population and follow-up
We identified 8574 potentially eligible women diagnosed with a primary invasive breast cancer (International Classification of Diseases-10 code C50). Of these, 703 (8.2%) patients were ineligible due to a prior invasive cancer diagnosis, 78 (0.9%) had non-epithelial tumors, one had a tumor that was not morphologically verified and five were registered as emigrating before their diagnosis date. Among 7787 remaining eligible women, we excluded 286 (3.7%) women (248 immigrants and 38 Norwegian) due to an unknown education or income level, leaving a final study population of 7501 breast cancer patients. Follow-up for survival started on the 15th of the month of breast cancer diagnosis and ended upon first emigration from Norway, death, after 12 years follow-up, or 31 December 2017, whichever came first.
Education level
We categorized patients by their most recently recorded education level before diagnosis: compulsory (lower secondary school, ≤ 10 years), secondary (upper secondary school or vocational education, 11–13 years) or tertiary (university or vocational education, ≥ 14 years). Our data included education level per 1 October 1999, 2000, 2005, 2010 and 2015. Norwegian educational institutions have mandatory reporting to the National Education Database. In our cohort, education level was 99.7% complete for Norwegian-born patients but was missing for 17.3% of eligible immigrants (2.0% of all eligible patients), most likely because these immigrants had not completed any education in Norway [23].
Income quintile
We divided patients into quintiles of average personal income during the five-year period before breast cancer diagnosis. We categorized patients by income before diagnosis since income is likely to fall after diagnosis [24]. We categorized income quintile (Q) as low (Q1), middle (Q2-Q4) or high (Q5). Our data included average annual income during 1995–1999, 2000–2004 and 2005–2009. We therefore divided patients diagnosed in 2000–2004 into quintiles of average income during 1995–1999, patients diagnosed in 2005–2009 into quintiles of average income during 2000–2004, and patients diagnosed in 2010–2015 into quintiles of average income during 2005–2009. Past income was 99.8% complete for Norwegian-born patients but was missing for 17.8% of eligible immigrants (2.1% of all eligible patients), probably because these immigrants did not reside in Norway during the period before diagnosis when income was recorded.
Socioeconomic status
We were interested in the effect of having both low education and low income, so formed a combined SES categorization of education and income level, where we separated the lowest education and income levels from higher levels. We divided patients into four SES groups: low/low (compulsory education/Q1 income), low/high (compulsory education/Q2–Q5 income), high/low (secondary or tertiary education/Q1 income) and high/high (secondary or tertiary education/Q2–Q5 income).
Covariates
We categorized immigration history as immigrant if patients were foreign-born with foreign-born parents, or Norwegian if otherwise. For patients diagnosed in 2005–2015, we had information on tumor grade (low = 1, medium = 2, high = 3–4) and status (positive or negative) of the estrogen receptor (ER), progesterone receptor (PR) and HER2. Criteria for determining ER, PR and HER2 status by the Cancer Registry of Norway are described elsewhere [15]. We combined information on ER, PR, HER2 and grade to classify clinical subtype as: luminal A-like (ER and/or PR positive, HER2 negative, low grade), luminal B-like/HER2− (ER and/or PR positive, HER2 negative, medium/high grade), luminal B-like/HER2+ (ER and/or PR positive, HER2 positive, any grade), HER2+ (ER and PR negative, HER2 positive, any grade) or triple-negative (ER and PR negative, HER2 negative, any grade) [25]. Subtype was set to unknown if any of ER, PR, HER2 or grade were missing.
Stage at diagnosis
We categorized tumor stage by pathological tumor size, nodal status and metastasis (TNM), supplemented with information from clinical reports of stage according to the Surveillance Epidemiology and End Results Program [1]. We categorized stage as localized (TNM stage I; tumors localized to the breast); regional (TNM stages II-III; metastasis to regional lymph nodes or to skin and/or chest wall); distant (TNM stage IV; metastasis to distant lymph nodes or other organs) or unknown (pathological and clinical reports were missing or incomplete). We combined TNM stages II and III because the coding practice for lymph node spread was updated at the Cancer Registry of Norway in 2008, leading to a migration between TNM stages II and III.
Statistical analysis
We used Pearson’s Chi-squared tests to determine associations between socioeconomic variables and covariates (tumor stage, age group, diagnostic period, immigration history and clinical subtype). Associations between socioeconomic variables and breast cancer death were determined by relative survival methods, which estimate excess mortality rates due to breast cancer by comparing the observed all-cause mortality rates of patients to the expected all-cause mortality rates for females in the Norwegian population of the same age and calendar year. In preliminary analyses, we used life tables stratified by age, calendar year and socioeconomic variables to avoid bias [26]. The SES-stratified life tables were created from individually linked nationwide data of mortality, education and income, and smoothed using a multivariable flexible Poisson model [27]. We found, however, that relative survival estimates were similar when using national life tables, so therefore used the simpler un-stratified national life tables in all analyses.
We first estimated stage-specific socioeconomic inequalities in excess mortality pooled over the study period (2000–2015). We used flexible parametric models [28, 29] to estimate stage-specific excess mortality rate ratios, with 95% confidence intervals (CI), by education, income and SES group, while adjusting for age and year at diagnosis. Immigration history and clinical subtype were assessed, but not included in final models because neither were important confounders or mediators of the main effects of education, income or SES group. In all models, the baseline hazard spline utilized four degrees of freedom and varied by stage at diagnosis with two degrees of freedom [28, 29]. Year at diagnosis was modeled non-linearly using restricted cubic splines with two degrees of freedom [30]. Modeling with splines allowed us to capture any changes in the rate of survival gain at a certain time points, for example after implementation of a new treatment. Three-way interactions between year, stage and socioeconomic variable allowed rates of survival gain to vary by both stage and socioeconomic group.
From these flexible parametric models, we made model-based predictions of 5-year relative survival with 95% confidence intervals (CI) for patients aged 40 years at diagnosis. We first predicted stage-specific 5-year relative survival over time for each socioeconomic group, then predicted difference in 5-year relative survival between the highest and lowest socioeconomic groups in 2000 and 2015. For patients diagnosed in 2015, we also made model-based predictions of relative survival up to 12 years from diagnosis. These 2015 predictions are outside the scope of the data and hence based on model parameters, so for comparison we calculated non-parametric Pohar Perme estimates of net survival [31] for patients diagnosed during 2005–2015 (Online Resource 1 and 2). The results were similar between model-based and non-parametric estimates.
We performed our analysis using STATA version 15.1 (StataCorp LLC, College Station, TX, USA, RRID:SCR_012763) [32]. We considered a two-sided p value less than 0.05 as statistically significant. Ethical approval was obtained from the Regional Committee for Medical and Health Research Ethics in Norway (Ref. 2013/2376). The dataset is managed in accordance with the European General Data Protection Regulation (GDPR).
Results
This study included 7501 patients, among whom we observed 1117 excess deaths due to breast cancer over 58418 person-years follow-up from diagnosis. There were 2317 (30.9%) patients with localized stage, 4457 (59.4%) with regional stage, 233 (3.1%) with distant stage, and 494 (6.6%) with unknown stage at diagnosis. Average follow-up per patient diagnosed with localized, regional, distant and unknown stage breast cancer was 8.3, 7.7, 3.4 and 7.8 years, respectively. High education was associated with more recent diagnosis (p < 0.001) and younger age at diagnosis (p < 0.001), while high income was associated with older age at diagnosis (p < 0.001) (Table 1). Neither education (p = 0.336) nor income (p = 0.376) were associated with tumor subtype.
Stage-specific excess mortality
In all socioeconomic groups, excess mortality rates were clearly highest at distant stage, but regional stage accounted for the greatest number of excess deaths, because of the high number of patients diagnosed at regional stage (Table 2). After adjustment for diagnosis age and year, excess mortality due to regional and distant stage breast cancer was significantly higher for compulsory versus tertiary educated patients, and for patients in the lowest and middle quintiles compared to the highest income quintile. There was a tendency for greater educational and income inequalities in excess mortality with more advanced stage at diagnosis.
Trends in stage-specific 5-year relative survival
Over time, regional and distant stage 5-year relative survival improved the least for patients with compulsory education and for patients in the lowest income quintile (Table 3, Fig. 1a and b). Patients with both low (compulsory) education and low (Q1) income had no improvement at all over time in 5-year relative survival from localized, regional or distant stage disease (Table 4, Fig. 1c). Educational and income differences in 5-year relative survival widened particularly over time for distant stage disease. Between 2000 and 2015, the difference in distant stage relative survival widened from 21 to 39% for tertiary versus compulsory educated patients, from 5 to 39% for patients in the highest versus lowest income quintiles, and from 10 to 47% for patients with high education and high income versus low education and low income.
Trends in regional and distant stage 5-year relative survival, for a compulsory and tertiary educated patients (n = 2873); b patients in income quintiles Q1 and Q5 (n = 1908); and c) patients with compulsory education/Q1 income and secondary-tertiary education/Q2-Q5 income (n = 3425). aModel-based predictions of relative survival, with 95% CI, for patients aged 40 years at diagnosis, compared to expected survival for the Norwegian female population. Note that predictions after 2012 are outside the scope of the data. bEducation/Income group: Low/Low = Compulsory/Income quintile Q1; High/High = Secondary-Tertiary/Income quintiles Q2–Q5
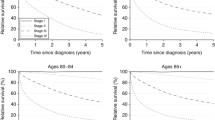
By 2015, model-based predictions of regional and distant stage relative survival were clearly better for tertiary and secondary compared to compulsory educated patients (Fig. 2a) and for patients in the highest income quintile versus the middle and lowest income quintiles (Fig. 2b). When education and income were examined in combination, we found both socioeconomic factors influenced regional stage relative survival, but education seemed a stronger predictor than income of distant stage relative survival (Fig. 2c). In 2015, the 5-year relative survival predictions for patients with low/low, low/high, high/low and high/high education/income level were, respectively: 98%, 97%, 98% and 99% for localized disease; 84%, 89%, 93% and 94% for regional stage disease; and 11%, 13%, 47% and 58% for distant stage disease (Table 4).
Model-based predictions of regional and distant stage relative survival for breast cancer patients aged 40 years at diagnosis in 2015, by a education level; b income quintile; and c education/income group (n = 7007). aRelative survival of patients compared to the expected survival for the Norwegian female population. Note that predictions beyond two years after diagnosis are outside the scope of the data, see Online Resource 1 for non-parametric relative survival curves. bEducation/Income group: Low/Low: Compulsory/Income quintile Q1; Low/High: Compulsory/ Income quintiles Q2–Q5; High/Low: Secondary–Tertiary/Q1; High/High: Secondary–Tertiary/Q2–Q5
Discussion
In Norway, a country with universal health care and national treatment guidelines, regional and distant stage survival improved more rapidly over time for young breast cancer patients with high SES compared to those with low SES. This widening survival gap over time between high and low SES patients was most pronounced for patients with distant spread at diagnosis. Survival from localized breast cancer was high for all socioeconomic groups throughout the study period, 2000–2015.
The reasons why low SES women lag behind high SES women in terms of survival gain are likely multifactorial. Potential reasons may include lifestyle, comorbidity, participation in clinical trials, differential access to new treatments, the opportunity or ability of patients to make informed treatment choices, motivation to adhere to treatment, or quality of care and follow-up provided by physicians. We and others [33, 34] have found no association between SES and tumor type, indicating that biological differences are unlikely to explain the association between SES and stage-specific survival.
Delayed access to new treatments may have delayed survival improvements for low SES patients [35]. There is evidence that differential treatment contributes to socioeconomic inequalities in survival, also in countries with universal health care [36, 37]. Despite universal health care and national treatment guidelines in Norway, high SES cancer patients have been reported to receive more hospital-based medical services [38] and more palliative radiotherapy [39], and high SES lung cancer patients have received more surgery and radiotherapy than low SES patients [40]. Similar surgical and radiotherapy differences have also been reported for breast cancer patients in the United Kingdom, where health care is also universal [41]. In Sweden, high SES patients were more likely to receive breast conserving surgery over mastectomy [42]. A recent study in the United Kingdom suggests that differential treatment contributes more to breast cancer survival inequalities than previously thought [36].
Scandinavian studies have found that comorbidity only plays a minor role in socioeconomic inequalities in breast cancer survival [43, 44]. On the other hand, lifestyle-related factors, such as overweight, smoking and alcohol, may partly explain poorer survival of low SES patients [44]. Unhealthy behavior has also been hypothesized to reduce the ability of low SES patients to respond to treatment [37]. If true, then a less healthy lifestyle may have potentially hindered low SES patients from benefitting from new treatments to the same extent as high SES patients.
For distant stage patients, education mattered more than income for survival. In a country with universal health care, survival inequalities may therefore not be about high SES patients affording better treatment, but about making better treatment choices. Particularly in the modern world, where treatment is becoming more personalized and complex, and the pros and cons must be continually weighed up by the patient and clinician. More educated patients may be more able to acquire knowledge about their diagnosis and take an active role in their treatment choices. A review of factors influencing socioeconomic inequalities in cancer survival [37] found that more affluent cancer patients communicated better with health care professionals than socioeconomically deprived patients. Affluent patients were also more likely to receive information from hospital specialists, had better psychological health and increased social support, which led to appropriate treatment being sought. Physicians may therefore need to pay more attention to socioeconomically deprived patients to ensure they receive equal access and standard of care [45].
Our findings of better regional and distant stage survival for high compared to low SES patients were in line with earlier studies of stage-specific survival from the USA [20], Netherlands [18] and Sweden [21]. However, we found no significant survival differences for localized disease, in contrast to earlier studies, possibly because we focused on young patients, whereas earlier studies included patients of all ages [18, 21] or only those over 55 years [20]. Nevertheless, our observation of better survival for high SES patients at regional and distant stage, where most deaths occurred, suggests that equal access to health care was not sufficient to offset any effect of SES on patient survival after diagnosis.
One important question is where there would be most to gain, by reducing SES differences in regional or distant stage survival? We found greater survival inequalities for distant stage patients than for regional stage patients, in line with earlier studies [18, 21]. However, twenty times more patients were diagnosed at regional stage compared to distant stage. The greatest number of deaths therefore occurred among patients with regional stage disease. Efforts to improve the regional stage survival of low SES patients would therefore be most effective for reducing breast cancer mortality in the population.
Our study had some limitations. We lacked information on lifestyle and treatment, so were not able to determine whether these factors explained our findings. Also, patient income was only available as five-year averages, so may not have reflected actual income at the time of diagnosis. However, income over five years may be reasonably correlated with accumulated disposable wealth at the time of diagnosis. Another potential study limitation was that some subgroups were small, particularly the number of distant stage patients. We nevertheless believe that our models for distant stage gave a good estimation of the true survival trends because we observed quite similar trends for regional stage, where patient numbers were much higher than for distant stage.
A major strength of our study was the population-wide registry data of high quality and completeness [22]. We had individually linked information on socioeconomic background and virtually complete follow-up of breast cancer patients for migration and death. Our life tables were constructed from individually linked demographic, migration and mortality data for the entire female Norwegian population. From an international perspective, Norwegian Cancer Registry data have high quality, with a very high proportion of morphologically verified cancers and a very small proportion identified through death certificate only [46], demonstrating high validity of our Cancer Registry data [47]. Further, a low proportion of our patient population had unknown stage at diagnosis, and the survival of these patients did not vary by SES. Thus, selection bias due to missing stage information was unlikely to explain our findings.
Conclusions
Despite Norway having universal health care and national treatment guidelines, we found that young breast cancer patients with low SES lag behind, with less improved regional and distant stage survival over time. Why socioeconomic status still matters for survival, even with equal health care access, is likely multifactorial and deserves more attention. Given the number of patients with regional stage disease, improving the survival of low SES patients with regional stage breast cancer would be most effective for reducing breast cancer mortality in the population.
Data availability
The data that support the findings of this study are available from the Cancer Registry of Norway but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors onsite at the Cancer Registry of Norway upon reasonable request and with permission of the Regional Committee for Medical and Health Research Ethics in Norway.
Abbreviations
- SES:
-
Socioeconomic status
- HER2:
-
Human epidermal growth factor receptor 2
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- Q:
-
Quintile
- TNM:
-
Tumor size, nodal status and metastasis
- CI:
-
Confidence interval
References
Larsen IK, Myklebust TA, Johannesen TB, Moller B, Hofvind S (2018) Stage-specific incidence and survival of breast cancer in Norway: the implications of changes in coding and classification practice. Breast 38:107–113. https://doi.org/10.1016/j.breast.2017.12.001
Crocetti E, Roche L, Buzzoni C, di Costanzo F, Molinie F, Caldarella A (2017) Trends in net survival from breast cancer in six European Latin countries: results from the SUDCAN population-based study. Eur J Cancer Prev 26:S85–s91. https://doi.org/10.1097/CEJ.0000000000000291
Bartlett JM, Brookes CL, Robson T, van de Velde CJ, Billingham LJ, Campbell FM, Grant M, Hasenburg A, Hille ET, Kay C, Kieback DG, Putter H, Markopoulos C, Kranenbarg EM, Mallon EA, Dirix L, Seynaeve C, Rea D (2011) Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: a prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial. J Clin Oncol 29(12):1531–1538
Regan MM, Pagani O, Francis PA, Fleming GF, Walley BA, Kammler R, Dell'Orto P, Russo L, Szoke J, Doimi F, Villani L, Pizzolitto S, Ohlschlegel C, Sessa F, Peg Camara V, Rodriguez Peralto JL, MacGrogan G, Colleoni M, Goldhirsch A, Price KN, Coates AS, Gelber RD, Viale G (2015) Predictive value and clinical utility of centrally assessed ER, PgR, and Ki-67 to select adjuvant endocrine therapy for premenopausal women with hormone receptor-positive, HER2-negative early breast cancer: TEXT and SOFT trials. Breast Cancer Res Treat 154(2):275–286. https://doi.org/10.1007/s10549-015-3612-z
Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN (2009) The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 14(4):320–368. https://doi.org/10.1634/theoncologist.2008-0230
Matsumoto A, Jinno H, Ando T, Fujii T, Nakamura T, Saito J, Takahashi M, Hayashida T, Kitagawa Y (2016) Biological markers of invasive breast cancer. Jpn J Clin Oncol 46(2):99–105. https://doi.org/10.1093/jjco/hyv153
Shepard HM, Jin P, Slamon DJ, Pirot Z, Maneval DC (2008) Herceptin. Handb Exp Pharmacol 181:183–219. https://doi.org/10.1007/978-3-540-73259-4_9
Slamon D, Pegram M (2001) Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials. Semin Oncol 28(1 Suppl 3):13–19. https://doi.org/10.1016/S0093-7754(01)90188-5
Dalton SO, Olsen MH, Johansen C, Olsen JH, Andersen KK (2019) Socioeconomic inequality in cancer survival—changes over time. A population-based study, Denmark, 1987–2013. Acta Oncol 58(5):737–744
Exarchakou A, Rachet B, Belot A, Maringe C, Coleman MP (2018) Impact of national cancer policies on cancer survival trends and socioeconomic inequalities in England, 1996–2013: population based study. BMJ 360:k764. https://doi.org/10.1136/bmj.k764
Stanbury JF, Baade PD, Yu Y, Yu XQ (2016) Cancer survival in New South Wales, Australia: socioeconomic disparities remain despite overall improvements. BMC Cancer 16:48. https://doi.org/10.1186/s12885-016-2065-z
Ito Y, Nakaya T, Nakayama T, Miyashiro I, Ioka A, Tsukuma H, Rachet B (2014) Socioeconomic inequalities in cancer survival: a population-based study of adult patients diagnosed in Osaka, Japan, during the period 1993–2004. Acta Oncol 53(10):1423–1433. https://doi.org/10.3109/0284186x.2014.912350
Kravdal H (2013) Widening educational differences in cancer survival in Norway. Eur J Pub Health 24(2):270–275. https://doi.org/10.1093/eurpub/ckt082
Soeberg M, Blakely T, Sarfati D (2015) Trends in ethnic and socioeconomic inequalities in cancer survival, New Zealand, 1991–2004. Cancer Epidemiol 39(6):860–862. https://doi.org/10.1016/j.canep.2015.10.018
Johansson ALV, Trewin CB, Hjerkind KV, Ellingjord-Dale M, Johannesen TB, Ursin G (2018) Breast cancer-specific survival by clinical subtype after seven years follow-up of young and elderly women in a nationwide cohort. Int J Cancer 144(6):1251–1261. https://doi.org/10.1002/ijc.31950
Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP, Weeks JC, Tamimi RM (2016) Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol 34(27):3308–3314
Verma R, Bowen RL, Slater SE, Mihaimeed F, Jones JL (2012) Pathological and epidemiological factors associated with advanced stage at diagnosis of breast cancer. Br Med Bull 103(1):129–145. https://doi.org/10.1093/bmb/lds018
Bastiaannet E, de Craen AJ, Kuppen PJ, Aarts MJ, van der Geest LG, van de Velde CJ, Westendorp RG, Liefers GJ (2011) Socioeconomic differences in survival among breast cancer patients in the Netherlands not explained by tumor size. Breast Cancer Res Treat 127(3):721–727. https://doi.org/10.1007/s10549-010-1250-z
Bower H, Andersson TM, Syriopoulou E, Rutherford MJ, Lambe M, Ahlgren J, Dickman PW, Lambert PC (2019) Potential gain in life years for Swedish women with breast cancer if stage and survival differences between education groups could be eliminated - Three what-if scenarios. Breast 45:75–81. https://doi.org/10.1016/j.breast.2019.03.005
Yabroff KR, Gordis L (2003) Does stage at diagnosis influence the observed relationship between socioeconomic status and breast cancer incidence, case-fatality, and mortality? Soc Sci Med 57(12):2265–2279. https://doi.org/10.1016/S0277-9536(03)00100-X
Rutqvist LE, Bern A (2006) Socioeconomic gradients in clinical stage at presentation and survival among breast cancer patients in the Stockholm area 1977–1997. Int J Cancer 119(6):1433–1439. https://doi.org/10.1002/ijc.21949
Larsen IK, Smastuen M, Johannesen TB, Langmark F, Parkin DM, Bray F, Moller B (2009) Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 45(7):1218–1231. https://doi.org/10.1016/j.ejca.2008.10.037
Statistics Norway (2013) Population's level of education, after the survey on education 2011/2012. https://www.ssb.no/en/utdanning/artikler-og-publikasjoner/befolkningens-utdanningsniva-etter-sporreundersokelsen-om-utdanning-fullfort-i-utlandet. Accessed 19 Oct 2018
Saltyte Benth J, Dahl FA, Luras H, Dahl AA (2014) A controlled study of income development for breast cancer survivors in Norway. Journal of cancer survivorship : research and practice 8(2):239–247. https://doi.org/10.1007/s11764-013-0324-4
Parise CA, Caggiano V (2014) Breast Cancer Survival Defined by the ER/PR/HER2 Subtypes and a Surrogate Classification according to Tumor Grade and Immunohistochemical Biomarkers. Journal of cancer epidemiology 2014:469251. https://doi.org/10.1155/2014/469251
Blakely T, Soeberg M, Carter K, Costilla R, Atkinson J, Sarfati D (2012) Bias in relative survival methods when using incorrect life-tables: lung and bladder cancer by smoking status and ethnicity in New Zealand. Int J Cancer 131(6):E974–982. https://doi.org/10.1002/ijc.27531
Rachet B, Maringe C, Woods LM, Ellis L, Spika D, Allemani C (2015) Multivariable flexible modelling for estimating complete, smoothed life tables for sub-national populations. BMC Public Health 15:1240. https://doi.org/10.1186/s12889-015-2534-3
Lambert PC, Royston P (2009) Further development of flexible parametric models for survival analysis. Stata Journal 9(2):265–290. https://doi.org/10.1177/1536867X0900900206
Nelson CP, Lambert PC, Squire IB, Jones DR (2007) Flexible parametric models for relative survival, with application in coronary heart disease. Stat Med 26(30):5486–5498. https://doi.org/10.1002/sim.3064
Durrleman S, Simon R (1989) Flexible regression models with cubic splines. Stat Med 8(5):551–561
Perme MP, Stare J, Esteve J (2012) On estimation in relative survival. Biometrics 68(1):113–120. https://doi.org/10.1111/j.1541-0420.2011.01640.x
StataCorp (2018) STATA statistical software [program]. 15.1 edn. Stata Corporation, College Station TX 77845, USA
Rutherford MJ, Hinchliffe SR, Abel GA, Lyratzopoulos G, Lambert PC, Greenberg DC (2013) How much of the deprivation gap in cancer survival can be explained by variation in stage at diagnosis: an example from breast cancer in the East of England. Int J Cancer 133(9):2192–2200. https://doi.org/10.1002/ijc.28221
McKenzie F, Ellison-Loschmann L, Jeffreys M (2010) Investigating reasons for socioeconomic inequalities in breast cancer survival in New Zealand. Cancer Epidemiol 34(6):702–708. https://doi.org/10.1016/j.canep.2010.07.007
Lyratzopoulos G, Barbiere JM, Rachet B, Baum M, Thompson MR, Coleman MP (2011) Changes over time in socioeconomic inequalities in breast and rectal cancer survival in England and Wales during a 32-year period (1973–2004): the potential role of health care. Ann Oncol 22(7):1661–1666. https://doi.org/10.1093/annonc/mdq647
Li R, Daniel R, Rachet B (2016) How much do tumor stage and treatment explain socioeconomic inequalities in breast cancer survival? Applying causal mediation analysis to population-based data. Eur J Epidemiol 31(6):603–611. https://doi.org/10.1007/s10654-016-0155-5
Woods LM, Rachet B, Coleman MP (2006) Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol 17(1):5–19. https://doi.org/10.1093/annonc/mdj007
Elstad JI (2018) Educational inequalities in hospital care for mortally ill patients in Norway. Scand J Public Health 46(1):74–82. https://doi.org/10.1177/1403494817705998
Asli LM, Myklebust TA, Kvaloy SO, Jetne V, Moller B, Levernes SG, Johannesen TB (2018) Factors influencing access to palliative radiotherapy: a Norwegian population-based study. Acta Oncol 57(9):1250–1258. https://doi.org/10.1080/0284186X.2018.1468087
Nilssen Y, Strand TE, Fjellbirkeland L, Bartnes K, Brustugun OT, O'Connell DL, Yu XQ, Moller B (2016) Lung cancer treatment is influenced by income, education, age and place of residence in a country with universal health coverage. Int J Cancer 138(6):1350–1360. https://doi.org/10.1002/ijc.29875
Downing A, Prakash K, Gilthorpe MS, Mikeljevic JS, Forman D (2007) Socioeconomic background in relation to stage at diagnosis, treatment and survival in women with breast cancer. Br J Cancer 96(5):836–840. https://doi.org/10.1038/sj.bjc.6603622
Frisell A, Lagergren J, Halle M, de Boniface J (2020) Socioeconomic status differs between breast cancer patients treated with mastectomy and breast conservation, and affects patient-reported preoperative information. Breast Cancer Res Treat 179(3):721–729. https://doi.org/10.1007/s10549-019-05496-2
Abdoli G, Bottai M, Sandelin K, Moradi T (2017) Breast cancer diagnosis and mortality by tumor stage and migration background in a nationwide cohort study in Sweden. Breast 31:57–65. https://doi.org/10.1016/j.breast.2016.10.004
Larsen SB, Kroman N, Ibfelt EH, Christensen J, Tjonneland A, Dalton SO (2015) Influence of metabolic indicators, smoking, alcohol and socioeconomic position on mortality after breast cancer. Acta Oncol 54(5):780–788. https://doi.org/10.3109/0284186x.2014.998774
Ibfelt EH, Dalton SO, Hogdall C, Fago-Olsen CL, Steding-Jessen M, Osler M, Johansen C, Frederiksen K, Kjaer SK (2015) Do stage of disease, comorbidity or access to treatment explain socioeconomic differences in survival after ovarian cancer? - A cohort study among Danish women diagnosed 2005–2010. Cancer Epidemiol 39(3):353–359. https://doi.org/10.1016/j.canep.2015.03.011
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, Bonaventure A, Valkov M, Johnson CJ, Esteve J, Ogunbiyi OJ, Azevedo ESG, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP (2018) Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391(10125):1023–1075. https://doi.org/10.1016/s0140-6736(17)33326-3
Bray F, Parkin DM (2009) Evaluation of data quality in the cancer registry: principles and methods. Part I: comparability, validity and timeliness. Eur J Cancer 45(5):747–755. https://doi.org/10.1016/j.ejca.2008.11.032
Acknowledgements
Open Access funding provided by University of Oslo (incl Oslo University Hospital). This study was completed as part of a PhD funded by the Norwegian National Advisory Unit for Women’s Health, Oslo University Hospital. The Norwegian Cancer Society (Grant Number 161326) and the Cancer Registry of Norway funded the data linkage and overhead costs.
Funding
This study was funded by the Norwegian Cancer Society under Grant 161326.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Cassia Bree Trewin declares that she has no conflict of interest. Anna Louise Viktoria Johansson declares that she has no conflict of interest. Kirsti Vik Hjerkind declares that she has no conflict of interest. Bjørn Heine Strand declares that he has no conflict of interest. Cecilie Essholt Kiserud declares that she has no conflict of interest. Giske Ursin declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Regional Committee for Medical and Health Research Ethics in Norway (Ref. 2013/2376) and with the 1964 Helsinki declaration and its later amendments.
Informed consent
This study uses data from national population and health registries. The Regional Committee for Medical and Health Research Ethics in Norway (Ref. 2013/2376) determined that informed consent was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trewin, C.B., Johansson, A.L.V., Hjerkind, K.V. et al. Stage-specific survival has improved for young breast cancer patients since 2000: but not equally. Breast Cancer Res Treat 182, 477–489 (2020). https://doi.org/10.1007/s10549-020-05698-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05698-z