Abstract
Purpose
Breast cancer-related lymphedema (BCRL) is caused by an interruption of the lymphatic system after breast cancer treatment. Lymphaticovenous anastomosis (LVA), by which one or more patent lymphatic collecting vessels are connected to subcutaneous veins, shows promising results. Postoperatively, the patency of these anastomosis can be evaluated; however, little is known concerning the long-term patency after LVA in patients with BCRL. The aim of this study was to analyse the long-term patency, quality of life (QoL) and arm circumference after LVA, and to explore differences between patent and non-patent anastomosis and its correlation with clinical improvement.
Methods
Twenty-five patients underwent indocyanine green (ICG) lymphography, lymph ICF-questionnaire, and arm circumference measurement preoperatively and 12 months after the LVA procedure.
Results
Seventy-six percent of the patients showed at least one patent anastomosis after 12 months. Quality of life according to the Lymph-ICF increased significantly (p < 0.000); however, arm circumference showed no significant decrease. Sixty-five percent discontinued wearing compression stockings. The patent anastomosis group, compared with the non-patent anastomosis group showed, without significance, more improvement in QoL, arm circumference, and discontinuation of compression stockings, as well as a lower rate of infections both pre- and postoperatively, a shorter duration of lymphedema preoperatively, and a higher rate of early lymphedema and ICG stage.
Conclusions
LVA showed an acceptable patency and positive correlation between a patent anastomosis and clinical improvement after 12 months. Further research with a larger study population is required to determine whether outcomes or patient characteristics significantly correlate with a patent anastomosis after LVA operation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secondary lymphedema in the upper extremity is caused by an interruption of the lymphatic system following cancer, trauma, injury, or infection. Breast cancer, the most frequent cancer among women, affects over 2 million women each year [1, 2]. Breast cancer treatment involving lymph node dissection or radiotherapy is the most common cause in developing upper extremity lymphedema, also known as breast cancer-related lymphedema (BCRL) [3, 4]. As a result, symptoms may negatively affect their quality of life (QoL) such as a swollen limb, pain, heaviness, and erysipelas [5,6,7]. Peripheral lymphedema can be classified clinically into severity stages according to the International Society of Lymphology (ISL) [8]. With the increasing breast cancer survival rate and the profound impact on QoL, lymphedema treatment is in high demand [5, 6, 9, 10].
Lymphedema treatment options aim to decrease symptoms and prevent the progression of chronic lymphedema. Complex decongestive therapy has an important role in alleviating symptoms of lymphedema [11,12,13,14]. However, as this standard treatment might be needed lifelong, super-microsurgical techniques have been introduced [15,16,17,18]. Koshima et al. performed the lymphaticovenous anastomosis (LVA) by which one or more patent lymphatic collecting vessels are connected to subcutaneous veins, thus restoring the physiological lymphatic drainage [19,20,21,22]. Using indocyanine green (ICG), a fluorescent marker, and a near-infrared camera, the lymphatic system can be visualised and dermal backflow patterns can be described by the so-called ICG stage [23]. These stages ranging from 0 to V describe ICG lymphography pattern changes from a normal linear pattern to an abnormal dermal backflow pattern in severe lymphedema (stage IV and V). After the lymphatic collecting vessels have been mapped, an artificial anastomosis can be made using the microscope [24,25,26]. Postoperatively, the patency of the anastomosis can be evaluated using the same ICG imaging technique. However, little is known concerning the long-term patency rate after LVA in patients with BCRL. Only few studies addressed the patency of LVA in upper extremity lymphedema [27, 28], and most of them examined lower extremity lymphedema in small study populations [27, 29,30,31]. Moreover, only few studies discussed the characteristics influencing the patency of LVA and concluded that more anastomosis per patient result in a higher chance of at least one patent anastomosis [28].
The aim of the current study was to analyse the long-term patency rate, QoL according to the Lymph-ICF, arm circumference, and compression stockings discontinuation after LVA, as well as exploring the differences between patients presenting patent or non-patent anastomosis and correlations with clinical improvement.
Patients and methods
This study included consecutive patients who underwent a lymphaticovenous anastomosis procedure for breast cancer-related lymphedema in Maastricht University Medical Centre between January 2016 and May 2018. Approval of the IRB was obtained (METC 2018-0869). The inclusion criteria were: adult female patients, breast cancer-related lymphedema, viable lymphatic collecting vessels seen with a near-infrared ICG camera, and a minimum of 12-month follow-up period after LVA surgery. Patients with incomplete follow-up were excluded. Demographic data were documented, assembled retrospectively and analysed. Preoperatively and 12 months postoperatively, all patients underwent ICG lymphography, filled in the Lymph ICF-questionnaire for quality of life recording, and their arm circumference was measured.
Surgical technique
The surgical LVA procedure as described by Koshima et al. was performed under local anaesthesia (bupivacaine hydrochloride 5 mg/ml with adrenaline 5 µg/ml) [19]. In brief, the lymphatic collecting vessels were located prior to the operation with the above-mentioned ICG lymphography. During the operation a small incision was made. Next, using a microscope, one or more anastomosis were performed between an adequate subcutaneous vein and a lymphatic collecting vessel using Ethilon 11-0. Finally, the patency of the anastomosis was checked intraoperatively and the skin was closed. In this study, patients were not allowed to wear compression stockings or receive manual lymphatic drainage (MLD) in the first month postoperatively in order to avoid harming the anastomosis. After that period, they could choose, in consultation with the plastic surgeon, whether to continue wearing the compression stockings or not, depending on the presence of subjective complaints and the presence of swelling in the arm. The plastic surgeon and skin therapist assessed if the MLD could be continued, reduced, or stopped.
Outcomes
The patency of the anastomosis and QoL 12 months after LVA were the primary outcomes. The secondary outcomes were arm volume changes and the discontinuation of compression stockings. All variables were obtained both preoperatively (except the patency of anastomosis) and 12 months postoperatively.
Patency measurement
Lymphatic system mapping and anastomosis examination was performed using a near-infrared camera (Fluobeam®; Fluoptics, Grenoble France) preoperatively and 12 months after LVA surgery. ICG 0.5%, 0.05 ml (PULSION® 25 mg for solution, PULSION Medical Systems SE, Feldkirchen, Germany) was injected intracutaneously in the second and fourth webspace of the affected arm. Preoperatively, after 20–30 min, viable lymph vessels for LVA were located. Postoperatively, the patency of the lymphaticovenous anastomosis was assessed immediately after injection with the NIRF camera located right above the scar on the skin to identify the anastomosis. A short massage at the level where the lymphatic vessel is, assists the spreading of ICG through the anastomosis for better appreciation of the patency [19, 25, 26, 32]. Postoperatively, the patency was scored as follows: patent, non-patent, or not seen. The assessment of the score was done by two independent researchers. Only a clear flush of ICG through the anastomosis was scored as patent.
Quality of life
Each patient completed the Dutch version of the Lymphedema International Classification of Functioning (LYMPH-ICF) questionnaire resulting in a VAS score from 0 to 100 to examine their quality of life preoperatively and 12 months after LVA surgery. The lower the score, the better the quality of life. An increase of more than nine or decrease of more than eleven points on this VAS score was considered statistically significant [33].
UEL index
The arm volume changes in terms of circumference were measured according to the Upper Extremity Lymphedema Index (UEL index) [34]. After circumference measurement at standardised landmarks on the arm (olecranon, 5 cm above and below olecranon, wrist, dorsum of the hand), the UEL index was calculated from these circumference points and BMI [35]. Patients had to remove their compression stockings 24 h prior to the follow-up moment in order to achieve a reliable measurement.
Statistical analysis
The patency rate and the discontinuation of compression stockings were indicated by percentages. For the QoL and the arm volume changes, a paired samples T test was used to examine effects pre- and postoperatively. Differences in outcome and characteristics between patent and non-patent anastomosis were only investigated exploratively by univariate analysis for the effect on patency of QoL, UEL index, age, BMI, and onset of lymphedema (ordinal variables), and a Fisher’s Exact Test for the effect of ICG stage, ISL stage, diabetes mellitus (DM), pre- and postoperative complications, and postoperative infection (categorical variables).
Results
Patients characteristics
Twenty-five female patients with a total of 47 anastomosis, and a mean of 1.9 anastomosis per patient, were included in the current study. Mean follow-up time was 14.8 months. In 3 patients, a postoperative wound infection occurred, which was successfully treated with antibiotics. The patients’ demographic characteristics are plotted in Table 1.
Patency
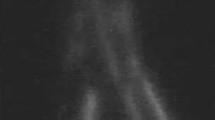
Postoperative examination with ICG lymphography demonstrated 19 out of 25 patients (76%) to have at least one patent anastomosis after 12 months. Figure 1 shows viable lymph vessels preoperatively and patent anastomosis postoperatively. In 2 patients, the anastomosis was not clearly seen and in 4 patients the anastomosis was non-patent. In total, 34 of 47 (72.3%) anastomosis were considered patent. An additional video of a patent anastomosis by ICG lymphography is given in Online Resource 1.
ICG lymphography with NIRF camera in 2 patients. Lymph vessels used for anastomosis are indicated by arrows, and location of the scar on the skin is indicated by curved lines. a Viable lymph vessels preoperatively and b non-patent anastomosis postoperatively (ICG flow stops at anastomosis site). c Viable lymph vessels preoperatively and d a patent anastomosis postoperatively
Quality of life
The Lymph-ICF questionnaire showed a significant improvement on hand function (p = 0.001), mental function (p = 0.002), and mobility (p = 0.006) 12 months after LVA surgery. The household and social domain did not differ significantly (p = 0.275 and p = 0.222, respectively). As a result, the difference in total score pre- and postoperatively also showed statistical significant improvement, 47.5 and 31.5, respectively (p < 0.000).
UEL index
The volume difference in terms of UEL index between the affected and unaffected arm was 16.2 preoperatively and 15.8 postoperatively, respectively. The difference, however, did not reach statistical significance (p = 0.822).
Conservative treatment
Frequencies of conservative treatment pre- and postoperatively are shown in Table 2. Preoperatively, 80% of the included patients wore compression stockings. Twelve months after LVA surgery, 65% of these patients completely stopped wearing the compression stockings and 10% wore them to a lesser degree.
Eighty-four percent of the patients were treated with MLD by a skin therapist preoperatively, with the frequency ranging from 3 times a week to once in every 6 weeks. After 12 months, 38% continued MLD in the same frequency, and 38% to a lesser degree. Twenty-four percent of the patients completely stopped MLD. Wearing of compression stockings and MLD frequency were never intensified. As a result, the mean frequency of MLD pre- and postoperatively was once per week and once in 2 weeks, respectively.
Patent vs. non-patent anastomosis
Differences in patients’ outcomes and characteristics between the patent and non-patent anastomosis groups are shown in Tables 3 and 4, respectively. Since none of the patients smoked and all diabetic patients showed a patent anastomosis, these variables were not analysed. Neither the outcomes nor patient characteristics between the patent and non-patent group showed significance after statistical analysis.
Discussion
In the current study, in three quarters of patients, at least one anastomosis was considered patent. Compared with the non-patent group, the patent anastomosis group showed more improvement in QoL, a decrease in arm circumference, and a higher discontinuation rate of compression stockings, indicating a conceivable positive correlation between a patent anastomosis and clinical improvement after LVA.
Only a few studies discussed the long-term patency rate, QoL, and arm circumference after LVA in the upper extremities. Recently, Winters et al. showed at least one patent anastomosis in 66.7% of 12 included patients [28]. Other studies examined the patency in the lower extremity after 6 to 12 months, with a patency rate ranging from 44 to 75% [29, 30, 36]. Mukenge et al. conducted LVA in 5 patients with genital lymphedema resulting in a patency rate of 100% after 12 months [31]. In these studies, similar imaging techniques (ICG lymphography) were used for evaluating the anastomosis showing different patterns of anastomosed veins, indicating that veins with different branching patterns can be used in the LVA procedure. Boccado et al. used the Kleinhand Transport Index after lymphoscintigraphy to evaluate the anastomosis [29]. Similar results were obtained on patency rate in the current study (76%).
Winters et al. described both a significant increase in QoL according to the LYMQoL and decrease in arm volume using the water displacement technique after a follow-up of 12 months [37]. Apart from the arm volume, these results are in line with the current study. Differences in results on arm volume may be explained by the different measuring techniques as this study measured the arm circumference instead of volume. Cornelissen et al. did not find a significant difference either, using the same technique [38]. Moreover, although only a slight decrease was presented in arm circumference, the current study showed a reduction in UEL index (− 2.1) in the patent group, while an increase in arm circumference was seen in the non-patent group (+ 4.2). Therefore, a patent anastomosis could have a positive impact on the arm circumference.
Three quarters of the patients with a patent anastomosis could discontinue the compression stockings. Twenty-five percent of the patients with non-patent anastomosis discontinued the compression stockings. This might seem as a high percentage; however, this concerned only one patient with ISL stage IV. Initially, this patient showed a decrease in arm circumference and symptoms, indicating a surgical benefit. Yet, after 1-year follow-up, the UEL index was increased and the anastomosis was shown to be non-patent.
Comparison between the number of anastomosis in relation to patency illustrated that in all patients with three anastomosis at least one was patent, whereas only 66.7% was patent in patients with only one anastomosis. Furthermore, the results presented an odds ratio of 3.7 between patent anastomosis and the number of anastomosis, suggesting a possible association between a higher number of anastomosis per patient and higher patency rate.
Many studies stated that LVA was less effective in advanced lymphedema and in patients presenting ICG stage IV or more [39,40,41,42,43]. Similarly, the current study illustrated a lower patency rate in ISL stage IIB and in ICG stage IV (OR 0.3 and OR 0.1, respectively). On the contrary, ISL stage IIA (OR 3.3) and ICG stage III (OR 16.5) showed a positive correlation with a patent anastomosis, indicating that early stage lymphedema could result in a higher chance of a patent anastomosis as it was postulated before by other authors [44,45,46].
Pre- and postoperative infections (both OR 0.1) may be associated with a lower chance of a patent anastomosis.
Due to the fact that patients consider the compression stockings as the greatest factor in decreasing QoL and previous studies showed a large discontinuation rate of compression stockings, this was considered one of the secondary outcomes [10, 32].
Postoperatively, MLD was continued in the same frequency, to a lesser degree, or stopped depending on symptoms and swelling of the arm. Therefore, outcomes achieved are a combination of LVA operation and conservative therapy, by maintenance of the arm after surgery if necessary.
Due to the small sample size, statistical analysis showed no significance for the previous described results. Therefore, the results can only be described exploratively.
Limitations
Although the study population was small (N = 25), this is one of the largest one compared to the previous literature till date. Despite the small study population, still a significant increase in QoL was found and difference in patent and non-patent groups could be seen. The power for this study, however, was achieved for analysis of the QoL.
Moreover, only four patients presented non-patent anastomosis. Although this low number could be considered as a good result after LVA procedure, statistical analysis was limited due to the noticeable difference in number of subjects per group (N = 19 in patent versus N = 4 in non-patent).
Many patients considered ICG injections uncomfortable, thereby refusing to have a postoperative ICG lymphography. These patients were excluded, and therefore, there may be a selection bias in this study.
Conclusions
Acceptable patency rate after LVA at 12 months was presented. A positive correlation between a patent anastomosis and clinical improvement in terms of QoL, arm circumference, and discontinuation of compression stockings was suggested. Early stage lymphedema and higher number of anastomoses could be associated with better patency rate. However, in the non-patent group, higher incidence of infections might be associated. None of these differences were found to be significant, but a trend could be seen.
Further research with prospective, larger study population is required to provide higher evidence for the correlation between the patent anastomosis after LVA procedure and clinical improvement.
Abbreviations
- BCRL:
-
breast cancer-related lymphedema
- QoL:
-
quality of life
- ISL:
-
International Society of Lymphology
- LVA:
-
lymphaticovenous anastomosis
- ICG:
-
indocyanine green
- MLD:
-
manual lymphatic drainage
- UEL:
-
upper extremity lymphedema
- DM:
-
diabetes mellitus
- OR:
-
odds ratio
- CI:
-
confidence interval
References
World Health Organization (2019) Breast cancer. https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/. Accessed 2 July 2019
Ahmed RL, Schmitz KH, Prizment AE, Folsom AR (2011) Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res Treat 130(3):981–991. https://doi.org/10.1007/s10549-011-1667-z
DiSipio T, Rye S, Newman B, Hayes S (2013) Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 14(6):500–515. https://doi.org/10.1016/s1470-2045(13)70076-7
Johnson AR, Kimball S, Epstein S, Recht A, Lin SJ, Lee BT, James TA, Singhal D (2019) Lymphedema incidence after axillary lymph node dissection: quantifying the impact of radiation and the lymphatic microsurgical preventive healing approach. Ann Plast Surg 82(4S Suppl 3):S234–S241. https://doi.org/10.1097/sap.0000000000001864
Grada AA, Phillips TJ (2017) Lymphedema: pathophysiology and clinical manifestations. J Am Acad Dermatol 77(6):1009–1020. https://doi.org/10.1016/j.jaad.2017.03.022
Penha TR, Botter B, Heuts EM, Voogd AC, von Meyenfeldt MF, van der Hulst RR (2016) Quality of life in patients with breast cancer-related lymphedema and reconstructive breast surgery. J Reconstr Microsurg 32(6):484–490. https://doi.org/10.1055/s-0036-1572538
Fu MR (2014) Breast cancer-related lymphedema: symptoms, diagnosis, risk reduction, and management. World J Clin Oncol 5(3):241–247. https://doi.org/10.5306/wjco.v5.i3.241
The Diagnosis and Treatment of Peripheral Lymphedema (2016) Consensus document of the International Society of Lymphology (2016). Lymphology 49(4):170–184
van der Waal D, Verbeek AL, den Heeten GJ, Ripping TM, Tjan-Heijnen VC, Broeders MJ (2015) Breast cancer diagnosis and death in the Netherlands: a changing burden. Eur J Public Health 25(2):320–324. https://doi.org/10.1093/eurpub/cku088
Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD (2012) The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol 36(3):237–248. https://doi.org/10.1016/j.canep.2012.02.007
Ezzo J, Manheimer E, McNeely ML, Howell DM, Weiss R, Johansson KI, Bao T, Bily L, Tuppo CM, Williams AF, Karadibak D (2015) Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd003475.pub2
Shao Y, Zhong DS (2017) Manual lymphatic drainage for breast cancer-related lymphoedema. Eur J Cancer Care. https://doi.org/10.1111/ecc.12517
McNeely ML, Peddle CJ, Yurick JL, Dayes IS, Mackey JR (2011) Conservative and dietary interventions for cancer-related lymphedema: a systematic review and meta-analysis. Cancer 117(6):1136–1148. https://doi.org/10.1002/cncr.25513
Mutidisciplinaire richtlijn Lymfoedeem. (2014). https://www.lymfoedeem.nl
Basta MN, Gao LL, Wu LC (2014) Operative treatment of peripheral lymphedema: a systematic meta-analysis of the efficacy and safety of lymphovenous microsurgery and tissue transplantation. Plast Reconstr Surg 133(4):905–913. https://doi.org/10.1097/PRS.0000000000000010
Cornelissen AJM, Beugels J, Ewalds L, Heuts EM, Keuter XHA, Piatkowski A, van der Hulst R, Qiu Shao SS (2018) Effect of lymphaticovenous anastomosis in breast cancer-related lymphedema: a review of the literature. Lymphat Res Biol 16(5):426–434. https://doi.org/10.1089/lrb.2017.0067
O’Brien BM, Mellow CG, Khazanchi RK, Dvir E, Kumar V, Pederson WC (1990) Long-term results after microlymphaticovenous anastomoses for the treatment of obstructive lymphedema. Plast Reconstr Surg 85(4):562–572
O’Brien BM, Sykes P, Threlfall GN, Browning FS (1977) Microlymphaticovenous anastomoses for obstructive lymphedema. Plast Reconstr Surg 60(2):197–211
Koshima I, Inagawa K, Urushibara K, Moriguchi T (2000) Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg 16(6):437–442. https://doi.org/10.1055/s-2006-947150
Pressman JL, Burtz MV, Shafer L (1964) Further observations related to direct communications between lymph nodes and veins. Surg Gynecol Obstet 119:984–990
Retik AB, Perlmutter AD, Harrison JH (1965) Communications between lymphatics and veins involving the portal circulation. Am J Surg 109:201–205
Threefoot SA, Kent WT, Hatchett BF (1963) Lymphaticovenous and lymphaticolymphatic communications demonstrated by plastic corrosion models of rats and by postmortem lymphangiography in man. J Lab Clin Med 61:9–22
Narushima M, Yamamoto T, Ogata F, Yoshimatsu H, Mihara M, Koshima I (2016) Indocyanine green Lymphography Findings in Limb Lymphedema. J Reconstr Microsurg 32(1):72–79. https://doi.org/10.1055/s-0035-1564608
Ogata F, Azuma R, Kikuchi M, Koshima I, Morimoto Y (2007) Novel lymphography using indocyanine green dye for near-infrared fluorescence labeling. Ann Plast Surg 58(6):652–655. https://doi.org/10.1097/01.sap.0000250896.42800.a2
Unno N, Inuzuka K, Suzuki M, Yamamoto N, Sagara D, Nishiyama M, Konno H (2007) Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J Vasc Surg 45(5):1016–1021. https://doi.org/10.1016/j.jvs.2007.01.023
Yamamoto T, Yamamoto N, Doi K, Oshima A, Yoshimatsu H, Todokoro T, Ogata F, Mihara M, Narushima M, Iida T, Koshima I (2011) Indocyanine green-enhanced lymphography for upper extremity lymphedema: a novel severity staging system using dermal backflow patterns. Plast Reconstr Surg 128(4):941–947. https://doi.org/10.1097/prs.0b013e3182268cd9
Shih HB, Shakir A, Nguyen DH (2016) Use of indocyanine green-SPY angiography for tracking lymphatic recovery after lymphaticovenous anastomosis. Ann Plast Surg 76(Suppl 3):S232–S237. https://doi.org/10.1097/SAP.0000000000000766
Winters H, Tielemans HJP, Verhulst AC, Paulus VAA, Slater NJ, Ulrich DJO (2019) The long-term patency of lymphaticovenular anastomosis in breast cancer-related lymphedema. Ann Plast Surg 82(2):196–200. https://doi.org/10.1097/sap.0000000000001674
Boccardo F, De Cian F, Campisi CC, Molinari L, Spinaci S, Dessalvi S, Talamo G, Campisi C, Villa G, Bellini C, Parodi A, Santi PL, Campisi C (2013) Surgical prevention and treatment of lymphedema after lymph node dissection in patients with cutaneous melanoma. Lymphology 46(1):20–26
Maegawa J, Yabuki Y, Tomoeda H, Hosono M, Yasumura K (2012) Outcomes of lymphaticovenous side-to-end anastomosis in peripheral lymphedema. J Vasc Surg 55(3):753–760. https://doi.org/10.1016/j.jvs.2011.08.062
Mukenge SM, Catena M, Negrini D, Ratti F, Moriondo A, Briganti A, Rigatti P, Cipriani F, Ferla G (2011) Assessment and follow-up of patency after lymphovenous microsurgery for treatment of secondary lymphedema in external male genital organs. Eur Urol 60(5):1114–1119. https://doi.org/10.1016/j.eururo.2010.11.020
Yamamoto T, Matsuda N, Doi K, Oshima A, Yoshimatsu H, Todokoro T, Ogata F, Mihara M, Narushima M, Iida T, Koshima I (2011) The earliest finding of indocyanine green lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: the modified dermal backflow stage and concept of subclinical lymphedema. Plast Reconstr Surg 128(4):314e–321e. https://doi.org/10.1097/prs.0b013e3182268da8
Devoogdt N, Van Kampen M, Geraerts I, Coremans T, Christiaens MR (2011) Lymphoedema Functioning, Disability and Health questionnaire (Lymph-ICF): reliability and validity. Phys Ther 91(6):944–957. https://doi.org/10.2522/ptj.20100087
Yamamoto N, Yamamoto T, Hayashi N, Hayashi A, Iida T, Koshima I (2016) Arm volumetry versus upper extremity lymphedema index: validity of upper extremity lymphedema index for body-type corrected arm volume evaluation. Ann Plast Surg 76(6):697–699. https://doi.org/10.1097/sap.0000000000000259
Yamamoto T, Yamamoto N, Hara H, Mihara M, Narushima M, Koshima I (2013) Upper extremity lymphedema index: a simple method for severity evaluation of upper extremity lymphedema. Ann Plast Surg 70(1):47–49. https://doi.org/10.1097/SAP.0b013e3182275d23
Suzuki Y, Sakuma H, Yamazaki S (2019) Comparison of patency rates of lymphaticovenous anastomoses at different sites for lower extremity lymphedema. J Vasc Surg Venous Lymphat Disord 7(2):222–227. https://doi.org/10.1016/j.jvsv.2018.10.022
Winters H, Tielemans HJP, Hameeteman M, Paulus VAA, Beurskens CH, Slater NJ, Ulrich DJO (2017) The efficacy of lymphaticovenular anastomosis in breast cancer-related lymphedema. Breast Cancer Res Treat 165(2):321–327. https://doi.org/10.1007/s10549-017-4335-0
Cornelissen AJM, Kool M, Lopez Penha TR, Keuter XHA, Piatkowski AA, Heuts E, van der Hulst R, Qiu SS (2017) Lymphatico-venous anastomosis as treatment for breast cancer-related lymphedema: a prospective study on quality of life. Breast Cancer Res Treat 163(2):281–286. https://doi.org/10.1007/s10549-017-4180-1
Chung JH, Baek SO, Park HJ, Lee BI, Park SH, Yoon ES (2019) Efficacy and patient satisfaction regarding lymphovenous bypass with sleeve-in anastomosis for extremity lymphedema. Arch Plast Surg 46(1):46–56. https://doi.org/10.5999/aps.2018.00773
Gennaro P, Gabriele G, Mihara M, Kikuchi K, Salini C, Aboh I, Cascino F, Chisci G, Ungari C (2016) Supramicrosurgical lymphatico-venular anastomosis (LVA) in treating lymphoedema: 36-months preliminary report. Eur Rev Med Pharmacol Sci 20(22):4642–4653
Ito R, Wu CT, Lin MC, Cheng MH (2016) Successful treatment of early-stage lower extremity lymphedema with side-to-end lymphovenous anastomosis with indocyanine green lymphography assisted. Microsurgery 36(4):310–315. https://doi.org/10.1002/micr.30010
Kung TA, Champaneria MC, Maki JH, Neligan PC (2017) Current Concepts in the Surgical Management of Lymphedema. Plast Reconstr Surg 139(4):1003e–1013e. https://doi.org/10.1097/prs.0000000000003218
Seki Y, Kajikawa A, Yamamoto T, Takeuchi T, Terashima T, Kurogi N (2018) Single lymphaticovenular anastomosis for early-stage lower extremity lymphedema treated by the superior-edge-of-the-knee incision method. Plast Reconstr Surg Glob Open 6(2):e1679. https://doi.org/10.1097/gox.0000000000001679
Abbaci M, Conversano A, De Leeuw F, Laplace-Builhe C, Mazouni C (2019) Near-infrared fluorescence imaging for the prevention and management of breast cancer-related lymphedema: a systematic review. Eur J Surg Oncol. https://doi.org/10.1016/j.ejso.2019.06.009
Seki Y, Kajikawa A, Yamamoto T, Takeuchi T, Terashima T, Kurogi N (2019) The dynamic-lymphaticovenular anastomosis method for breast cancer treatment-related lymphedema: creation of functional lymphaticovenular anastomoses with use of preoperative dynamic ultrasonography. J Plastic Reconstr Aesthet Surg 72(1):62–70. https://doi.org/10.1016/j.bjps.2018.09.005
Liu HL, Pang SY, Chan YW (2014) The use of a microscope with near-infrared imaging function in indocyanine green lymphography and lymphaticovenous anastomosis. J Plast Reconstr Aesthet Surg 67(2):231–236. https://doi.org/10.1016/j.bjps.2013.10.039
Funding
The authors declare that they did not receive funding for this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wolfs, J.A.G.N., de Joode, L.G.E.H., van der Hulst, R.R.W.J. et al. Correlation between patency and clinical improvement after lymphaticovenous anastomosis (LVA) in breast cancer-related lymphedema: 12-month follow-up. Breast Cancer Res Treat 179, 131–138 (2020). https://doi.org/10.1007/s10549-019-05450-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05450-2