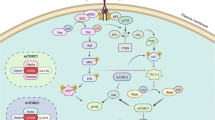
Abstract
Purpose
Recent studies have emphasized a key role for the anti-apoptotic Bcl-2 family member Mcl-1 in conferring tumor cell survival and drug resistance in breast cancer (BC). Mcl-1 inhibitors, such as the BH3-mimetic EU-5346, therefore represent an exciting new class of targeting agents and are a current focus of widespread cancer-drug development efforts.
Methods
ONCOMINE analysis was utilized to compare expression profiles of Bcl-2 family members across all major BC subgroups. Potential toxicities of EU-5346 were evaluated using iPS-generated cardiomyocytes, blood cells and astrocytes. The anti-BC cell activity of EU-5346-based therapies was evaluated using [3H]-thymidine uptake and spheroid-forming assays as well as immunoblotting and the Chou-Talalay method. Protein level-based activity of EU-5346, the specific anti-Bcl-2 inhibitor ABT-199 and the specific anti-Bcl-xL inhibitor WEHI-539 was verified in Mcl-1Δ/null versus Mcl-1wt/wt MEFs.
Results
We previously demonstrated significant anti-tumor activity of EU-5346 in all BC subtypes. Our present results go further and suggest that EU-5346 may induce limited adverse events such as cardiotoxicity, hematotoxicity, and neurotoxicity, frequently observed with other BH3 mimetics. As demonstrated by our mathematical scoring model, the prediction of EU-5643-induced IC50 not only relies on the protein level of Mcl-1 but also on Bak, Bim, and Noxa. Synergistic anti-BC activity of low-dose EU-5346 with the BH3 mimetics ABT-199 or WEHI-539 was observed only in those BC cells expressing Bcl-2 or Bcl-xL, respectively. Similarly, when combined with tamoxifen or trastuzumab, low-dose EU-5346 induced significant anti-BC activity in hormone receptor positive or Her2-positive BC cells, respectively. Finally, EU-5346 in combination with paclitaxel induced synergistic anti-BC activity in both paclitaxel-sensitive and paclitaxel-resistant TNBC cells.
Conclusion
These data strongly support the further clinical development of EU-5346 to improve BC patient survival.








Similar content being viewed by others
Abbreviations
- BC:
-
Breast cancer
- TN:
-
Triple negative
- MOMP:
-
Mitochondrial outer membrane permeabilization
- Mcl-1:
-
Myeloid cell leukemia-1
- BH3:
-
Bcl-2 homology 3
- TCGA:
-
The Cancer Genome Atlas (TCGA)
- HR:
-
Hormone receptor
- iPS:
-
Induced pluripotent stem cell
- MEFs:
-
Murine embryonic fibroblasts
- PBMCs:
-
Peripheral blood mononuclear cells
References
Network TCGA (2012) Comprehensive molecular portraits of human breast tumours. Nature 490:61–70. https://doi.org/10.1038/nature11412
Adams JM, Cory S (2007) The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26:1324–1337. https://doi.org/10.1038/sj.onc.1210220
Vogler M, Dinsdale D, Dyer MJS, Cohen GM (2009) Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ 16:360–367. https://doi.org/10.1038/cdd.2008.137
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Montero J, Letai A (2018) Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Diff 25:56–64. https://doi.org/10.1038/cdd.2017.183
Thomas LW, Lam C, Edwards SW (2010) Mcl-1; the molecular regulation of protein function. FEBS Lett 584:2981–2989. https://doi.org/10.1016/j.febslet.2010.05.061
Germain M, Duronio V (2007) The N terminus of the anti-apoptotic BCL-2 homologue MCL-1 regulates its localization and function. J Biol Chem 282:32233–32242. https://doi.org/10.1074/jbc.M706408200
Yang T, Kozopas KM, Craig RW (1995) The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical to, those of Bcl-2. J Cell Biol 128:1173–1184
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619
Del Gaizo Moore V, Letai A (2013) BH3 profiling–measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett 332:202–205. https://doi.org/10.1016/j.canlet.2011.12.021
Oda E, Ohki R, Murasawa H et al (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053–1058
Wei G, Margolin AA, Haery L et al (2012) Chemical genomics identifies small-molecule MCL1 repressors and BCL-xL as a predictor of MCL1 dependency. Cancer Cell 21:547–562. https://doi.org/10.1016/j.ccr.2012.02.028
Beroukhim R, Mermel CH, Porter D et al (2010) The landscape of somatic copy-number alteration across human cancers. Nature 463:899–905. https://doi.org/10.1038/nature08822
Leverson JD, Phillips DC, Mitten MJ et al (2015) Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med 7:279ra40. https://doi.org/10.1126/scitranslmed.aaa4642
Leverson JD, Zhang H, Chen J et al (2015) Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis 6:e1590. https://doi.org/10.1038/cddis.2014.561
Abulwerdi F, Liao C, Liu M et al (2014) A novel small-molecule inhibitor of mcl-1 blocks pancreatic cancer growth in vitro and in vivo. Mol Cancer Ther 13:565–575. https://doi.org/10.1158/1535-7163.MCT-12-0767
Kotschy A, Szlavik Z, Murray J et al (2016) The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 538:477–482. https://doi.org/10.1038/nature19830
Belmar J, Fesik SW (2015) Small molecule Mcl-1 inhibitors for the treatment of cancer. Pharmacol Ther 145:76–84. https://doi.org/10.1016/j.pharmthera.2014.08.003
Nguyen M, Marcellus RC, Roulston A et al (2007) Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci USA 104:19512–19517. https://doi.org/10.1073/pnas.0709443104
Richard DJ, Lena R, Bannister T et al (2013) Hydroxyquinoline-derived compounds and analoguing of selective Mcl-1 inhibitors using a functional biomarker. Bioorg Med Chem 21:6642–6649. https://doi.org/10.1016/j.bmc.2013.08.017
Bashari MH, Fan F, Vallet S et al (2016) Mcl-1 confers protection of Her2-positive breast cancer cells to hypoxia: therapeutic implications. Breast Cancer Res 18:26. https://doi.org/10.1186/s13058-016-0686-4
Ding Q, He X, Hsu J-M et al (2007) Degradation of Mcl-1 by beta-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol Cell Biol 27:4006–4017. https://doi.org/10.1128/MCB.00620-06
Campbell KJ, Dhayade S, Ferrari N et al (2018) MCL-1 is a prognostic indicator and drug target in breast cancer. Cell Death Dis 9:19. https://doi.org/10.1038/s41419-017-0035-2
Williams MM, Lee L, Hicks DJ et al (2017) Key survival factor, Mcl-1, correlates with sensitivity to combined Bcl-2/Bcl-xL blockade. Mol Cancer Res 15:259–268. https://doi.org/10.1158/1541-7786.MCR-16-0280-T
Merino D, Whittle JR, Vaillant F et al (2017) Synergistic action of the MCL-1 inhibitor S63845 with current therapies in preclinical models of triple-negative and HER2-amplified breast cancer. Sci Transl Med 9:eaam7049. https://doi.org/10.1126/scitranslmed.aam7049
Wertz IE, Kusam S, Lam C et al (2011) Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 471:110–114. https://doi.org/10.1038/nature09779
Placzek WJ, Wei J, Kitada S et al (2010) A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis 1:e40. https://doi.org/10.1038/cddis.2010.18
Booy EP, Henson ES, Gibson SB (2011) Epidermal growth factor regulates Mcl-1 expression through the MAPK-Elk-1 signalling pathway contributing to cell survival in breast cancer. Oncogene 30:2367–2378. https://doi.org/10.1038/onc.2010.616
van Delft MF, Wei AH, Mason KD et al (2006) The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10:389–399. https://doi.org/10.1016/j.ccr.2006.08.027
Oltersdorf T, Elmore SW, Shoemaker AR et al (2005) An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435:677–681. https://doi.org/10.1038/nature03579
Tse C, Shoemaker AR, Adickes J et al (2008) ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68:3421–3428. https://doi.org/10.1158/0008-5472.CAN-07-5836
Opferman JT, Letai A, Beard C et al (2003) Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426:671–676
Podar K, Gouill SL, Zhang J et al (2008) A pivotal role for Mcl-1 in bortezomib-induced apoptosis. Oncogene 27:721–731
Rhodes DR, Kalyana-Sundaram S, Mahavisno V et al (2007) Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 9:166–180
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. https://doi.org/10.1016/j.cell.2007.11.019
Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA (2009) Spheroid-based drug screen: considerations and practical approach. Nat Protoc 4:309–324. https://doi.org/10.1038/nprot.2008.226
Chou T-C (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70:440–446. https://doi.org/10.1158/0008-5472.CAN-09-1947
Bannister T, Koenig M, He Y, Mishra J, Spicer T, Minond D, Saldanha A, Mercer BA, Cameron M, Lena R, Carlson N, Richard D, Cardone MHP (2013) ML311: A Small Molecule that Potently and Selectively Disrupts the Protein-Protein Interaction of Mcl-1 and Bim: A Probe for Studying Lymphoid Tumo… In: Probe Reports from NIH Mol. Libr. Progr. [Internet]. Bethesda Natl. Cent. Biotechnol. Inf. http://www.ncbi.nlm.nih.gov/pubmed/23762927. Accessed 8 Jun 2015
Wang X, Bathina M, Lynch J et al (2013) Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction. Genes Dev 27:1351–1364. https://doi.org/10.1101/gad.215855.113
Nikhil K, Shah K (2017) The Cdk5-Mcl-1 axis promotes mitochondrial dysfunction and neurodegeneration in a model of Alzheimer’s disease. J Cell Sci 130:3023–3039. https://doi.org/10.1242/jcs.205666
Perciavalle RM, Stewart DP, Koss B et al (2012) Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol 14:575–583. https://doi.org/10.1038/ncb2488
Goodwin CM, Rossanese OW, Olejniczak ET, Fesik SW (2015) Myeloid cell leukemia-1 is an important apoptotic survival factor in triple-negative breast cancer. Cell Death Diff 22:2098–2106. https://doi.org/10.1038/cdd.2015.73
Zhang Z, Liu Y, Song T et al (2013) An antiapoptotic Bcl-2 family protein index predicts the response of leukaemic cells to the pan-Bcl-2 inhibitor S1. Br J Cancer 108:1870–1878. https://doi.org/10.1038/bjc.2013.152
Deng J, Carlson N, Takeyama K et al (2007) BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell 12:171–185. https://doi.org/10.1016/j.ccr.2007.07.001
Wei S-H, Dong K, Lin F et al (2008) Inducing apoptosis and enhancing chemosensitivity to gemcitabine via RNA interference targeting Mcl-1 gene in pancreatic carcinoma cell. Cancer Chemother Pharmacol 62:1055–1064. https://doi.org/10.1007/s00280-008-0697-7
Konopleva M, Contractor R, Tsao T et al (2006) Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10:375–388. https://doi.org/10.1016/j.ccr.2006.10.006
Goldsmith KC, Lestini BJ, Gross M et al (2010) BH3 response profiles from neuroblastoma mitochondria predict activity of small molecule Bcl-2 family antagonists. Cell Death Differ 17:872–882. https://doi.org/10.1038/cdd.2009.171
Morales AA, Kurtoglu M, Matulis SM et al (2011) Distribution of Bim determines Mcl-1 dependence or codependence with Bcl-xL/Bcl-2 in Mcl-1-expressing myeloma cells. Blood 118:1329–1339. https://doi.org/10.1182/blood-2011-01-327197
Al-Harbi S, Hill BT, Mazumder S et al (2011) An antiapoptotic BCL-2 family expression index predicts the response of chronic lymphocytic leukemia to ABT-737. Blood 118:3579–3590. https://doi.org/10.1182/blood-2011-03-340364
Goodwin CM, Rossanese OW, Olejniczak ET, Fesik SW (2015) Myeloid cell leukemia-1 is an important apoptotic survival factor in triple-negative breast cancer. Cell Death Differ. https://doi.org/10.1038/cdd.2015.73
Balko JM, Giltnane JM, Wang K et al (2014) Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov 4:232–245. https://doi.org/10.1158/2159-8290.CD-13-0286
Acknowledgements
SM is the recipient of a DGHO/ Jose Carreras stipend. KP is the recipient of a B. Braun Stiftungs Grant. MP and KP received research support from Roche Pharmaceuticals. We cordially thank Muhammad Hasan Bashari for technical assistance.
Author information
Authors and Affiliations
Contributions
SV conceived of the study, designed experiments, analyzed data, and wrote the manuscript. FF and SM performed experiments and participated in data analysis and interpretation. MS, JTO and MHC conceived of the study and participated in data analysis and interpretation. MP, AS and DJ made substantial contributions to the acquisition and interpretation of data. KP conceived of the study, designed and coordinated experiments, analyzed and interpreted data and wrote the manuscript. All authors were involved in revising the manuscript critically for important intellectual content. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
KP received speaker honorarium from Celgene, Janssen, and Amgen. MP and KP received research support from Roche Pharmaceuticals. JTO received consultant honorarium and research support from AbbVie. DJ received consultant honorarium from Bayer, Amgen, MSD, CureVac, Roche, BMS. MHC is the co-funder, president and CEO of Eutropics, Inc. The remaining authors declare no conflict of interest.
Ethical approval
This study complied with current laws of Germany, Austria and USA. The collection and use of primary cells has been approved by the Ethics committee of the Medical Faculty, University of Heidelberg (Approval Number 022/2013).
Informed consent
Informed consent was obtained in accordance with the Declaration of Helsinki. This article does not contain any studies with animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vallet, S., Fan, F., Malvestiti, S. et al. Rationally derived drug combinations with the novel Mcl-1 inhibitor EU-5346 in breast cancer. Breast Cancer Res Treat 173, 585–596 (2019). https://doi.org/10.1007/s10549-018-5022-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-5022-5