Abstract
Purpose
Ductal carcinoma in situ (DCIS) is a pre-invasive lesion of the breast considered a precursor of invasive ductal carcinoma. This study aimed to determine whether activated PPARγ acts as a tumor suppressor in human DCIS progression.
Methods
We utilized the high-affinity PPARγ agonist, efatutazone, to activate endogenous PPARγ in a well-defined model for the progression of basal (triple negative) DCIS, MCFDCIS cells, cultured under 2D and 3D conditions. We studied the effects of activated PPARγ on DCIS progression in MCFDCIS xenograft and C3(1)/Tag transgenic mice treated with 30 mg/kg of efatutazone.
Results
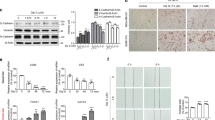
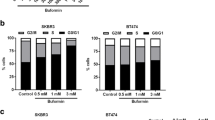
In vitro, efatutazone did not alter the MCFDCIS cell proliferation but induced phenotypic and gene expression changes, indicating that activated PPARγ is able to differentiate MCFDCIS cells into more luminal and lactational-like cells. In addition, MCFDCIS tumorsphere formation in 3D was reduced by PPARγ activation. In vivo, efatutazone-treated MCFDCIS tumors exhibited fat deposition along with upregulation of PPARγ responsive genes in both epithelial and stromal compartments, suggesting features of milk-producing mammary epithelial cell differentiation. The efatutazone-treated lesions were less invasive with fewer CD44+/p63+ basal progenitor cells. PPARγ activation downregulated Akt phosphorylation in these tumors, although the ERK pathway remained unchanged. Similar trends in gene expression changes consistent with lactational and luminal cell differentiation were observed in the C3(1)/Tag mouse model after efatutazone treatment.
Conclusions
Our data suggest that activation of the PPARγ pathway differentiates DCIS lesions and may be a useful approach to delay DCIS progression.






Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30. https://doi.org/10.3322/caac.21332
Wellings SR, Jensen HM (1973) On the origin and progression of ductal carcinoma in the human breast. J Natl Cancer Inst 50:1111–1118
Carter CL, Corle DK, Micozzi MS et al (1988) A prospective-study of the development of breast-cancer in 16,692 women with benign breast disease. Am J Epidemiol 128:467–477
Kuerer HM, Albarracin CT, Yang WT et al (2009) Ductal carcinoma in situ: state of the science and roadmap to advance the field. J Clin Oncol 27:279–288. https://doi.org/10.1200/JCO.2008.18.3103
O’Connell P, Pekkel V, Fuqua SA et al (1998) Analysis of loss of heterozygosity in 399 premalignant breast lesions at 15 genetic loci. J Natl Cancer Inst 90:697–703
Hwang ES, DeVries S, Chew KL et al (2004) Patterns of chromosomal alterations in breast ductal carcinoma in situ. Clin Cancer Res 10:5160–5167. https://doi.org/10.1158/1078-0432.CCR-04-0165
Buerger H, Simon R, Schäfer KL et al (2000) Genetic relation of lobular carcinoma in situ, ductal carcinoma in situ, and associated invasive carcinoma of the breast. MP, Mol Pathol 53:118–121
Rosen PP, Braun DW, Kinne DE (1980) The clinical significance of pre-invasive breast carcinoma. Cancer 46:919–925
Eusebi V, Feudale E, Foschini MP et al (1994) Long-term follow-up of in situ carcinoma of the breast. Semin Diagn Pathol 11:223–235
Page DL, Dupont WD, Rogers LW et al (1995) Continued local recurrence of carcinoma 15-25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer 76:1197–1200
Collins LC, Tamimi RM, Baer HJ et al (2005) Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses’ Health Study. Cancer 103:1778–1784. https://doi.org/10.1002/cncr.20979
Erbas B, Provenzano E, Armes J, Gertig D (2006) The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat 97:135–144. https://doi.org/10.1007/s10549-005-9101-z
Kietzman W, Riegel AT, Ory V (2017) Early-stage progression of breast cancer. In: Breast cancer—from biology to medicine, chap 4. InTech, pp 61–96
Michalik L, Desvergne B, Wahli W (2004) Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer 4:61–70. https://doi.org/10.1038/nrc1254
Marlow LA, Reynolds LA, Cleland AS et al (2009) Reactivation of suppressed RhoB is a critical step for the inhibition of anaplastic thyroid cancer growth. Cancer Res 69:1536–1544. https://doi.org/10.1158/0008-5472.CAN-08-3718
Copland JA, Marlow LA, Kurakata S et al (2005) Novel high-affinity PPARγ agonist alone and in combination with paclitaxel inhibits human anaplastic thyroid carcinoma tumor growth via p21WAF1/CIP1. Oncogene 25:2304–2317. https://doi.org/10.1038/sj.onc.1209267
Motomura W, Okumura T, Takahashi N et al (2000) Activation of peroxisome proliferator-activated receptor gamma by troglitazone inhibits cell growth through the increase of p27KiP1 in human. Pancreatic carcinoma cells. Cancer Res 60:5558–5564
Nicol CJ, Yoon M, Ward JM et al (2004) PPARgamma influences susceptibility to DMBA-induced mammary, ovarian and skin carcinogenesis. Carcinogenesis 25:1747–1755. https://doi.org/10.1093/carcin/bgh160
Yin Y, Russell RG, Dettin LE et al (2005) Peroxisome proliferator-activated receptor delta and gamma agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Res 65:3950–3957. https://doi.org/10.1158/0008-5472.CAN-04-3990
Yin Y, Yuan H, Zeng X et al (2009) Inhibition of peroxisome proliferator-activated receptor gamma increases estrogen receptor-dependent tumor specification. Cancer Res 69:687–694. https://doi.org/10.1158/0008-5472.CAN-08-2446
Suh N, Wang Y, Williams CR et al (1999) A new ligand for the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), GW7845, inhibits rat mammary carcinogenesis. Cancer Res 59:5671–5673
Kulke MH, Demetri GD, Sharpless NE et al (2002) A phase II study of troglitazone, an activator of the PPARgamma receptor, in patients with chemotherapy-resistant metastatic colorectal cancer. Cancer J 8:395–399
Smith MR, Manola J, Kaufman DS et al (2004) Rosiglitazone versus placebo for men with prostate carcinoma and a rising serum prostate-specific antigen level after radical prostatectomy and/or radiation therapy. Cancer 101:1569–1574. https://doi.org/10.1002/cncr.20493
Pishvaian MJ, Marshall JL, Wagner AJ et al (2012) A phase 1 study of efatutazone, an oral peroxisome proliferator-activated receptor gamma agonist, administered to patients with advanced malignancies. Cancer 118:5403–5413. https://doi.org/10.1002/cncr.27526
Nakles RE, Kallakury BVS, Furth PA (2013) The PPARγ agonist efatutazone increases the spectrum of well-differentiated mammary cancer subtypes initiated by loss of full-length BRCA1 in association with TP53 haploinsufficiency. Am J Pathol 182:1976–1985. https://doi.org/10.1016/j.ajpath.2013.02.006
Miller FR, Santner SJ, Tait L, Dawson PJ (2000) MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst 92:1185–1186
Hu M, Yao J, Carroll DK et al (2008) Regulation of in situ to invasive breast carcinoma transition. Cancer Cell 13:394–406. https://doi.org/10.1016/j.ccr.2008.03.007
Ory V, Tassi E, Cavalli LR et al (2014) The nuclear coactivator amplified in breast cancer 1 maintains tumor-initiating cells during development of ductal carcinoma in situ. Oncogene 33:3033–3042. https://doi.org/10.1038/onc.2013.263
Debnath J, Muthuswamy SK, Brugge JS (2003) Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30:256–268. https://doi.org/10.1016/S1046-2023(03)00032-X
Lee GY, Kenny PA, Lee EH, Bissell MJ (2007) Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Meth 4:359–365. https://doi.org/10.1038/nmeth1015
Berens EB, Sharif GM, Schmidt MO et al (2017) Keratin-associated protein 5-5 controls cytoskeletal function and cancer cell vascular invasion. Oncogene 36:593–605. https://doi.org/10.1038/onc.2016.234
Green JE, Shibata MA, Yoshidome K et al (2000) The C3(1)/SV40 T-antigen transgenic mouse model of mammary cancer: ductal epithelial cell targeting with multistage progression to carcinoma. Oncogene 19:1020–1027. https://doi.org/10.1038/sj.onc.1203280
Al-Otaiby M, Tassi E, Schmidt M et al (2012) Role of the nuclear receptor coactivator AIB1/SRC-3 in angiogenesis and wound healing. Am J Pathol 180:1474–1484. https://doi.org/10.1016/j.ajpath.2011.12.032
Fereshteh MP, Tilli MT, Kim SE et al (2008) The nuclear receptor coactivator amplified in breast cancer-1 is required for Neu (ErbB2/HER2) activation, signaling, and mammary tumorigenesis in mice. Cancer Res 68:3697–3706. https://doi.org/10.1158/0008-5472.CAN-07-6702
Oh A, List H-J, Reiter R et al (2004) The nuclear receptor coactivator AIB1 mediates insulin-like growth factor I-induced phenotypic changes in human breast cancer cells. Cancer Res 64:8299–8308. https://doi.org/10.1158/0008-5472.CAN-04-0354
Girousse A, Langin D (2012) Adipocyte lipases and lipid droplet-associated proteins: insight from transgenic mouse models. Int J Obes (Lond) 36:581–594. https://doi.org/10.1038/ijo.2011.113
Mather IH, Keenan TW (1998) Origin and secretion of milk lipids. J Mammary Gland Biol Neoplasia 3:259–273
McManaman JL (2009) Formation of milk lipids: a molecular perspective. Clin Lipidol 4:391–401. https://doi.org/10.2217/clp.09.15
Anders C, Carey LA (2008) Understanding and treating triple-negative breast cancer. Oncology (Williston Park, NY) 22:1233–9– discussion 1239–40– 1243
Shimazaki N, Togashi N, Hanai M et al (2008) Anti-tumour activity of CS-7017, a selective peroxisome proliferator-activated receptor gamma agonist of thiazolidinedione class, in human tumour xenografts and a syngeneic tumour implant model. Eur J Cancer 44:1734–1743. https://doi.org/10.1016/j.ejca.2008.04.016
Gebhardt C, Németh J, Angel P, Hess J (2006) S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol 72:1622–1631. https://doi.org/10.1016/j.bcp.2006.05.017
Chakraborty S, Kaur S, Guha S, Batra SK (2012) The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta 1826:129–169. https://doi.org/10.1016/j.bbcan.2012.03.008
Bach LA, Fu P, Yang Z (2013) Insulin-like growth factor-binding protein-6 and cancer. Clin Sci 124:215–229. https://doi.org/10.1042/CS20120343
Sawayama H, Ishimoto T, Watanabe M et al (2014) Small molecule agonists of PPAR-γ exert therapeutic effects in esophageal cancer. Cancer Res 74:575–585. https://doi.org/10.1158/0008-5472.CAN-13-1836
Qin L, Ren Y, Chen A-M et al (2014) Peroxisome proliferator-activated receptor γ ligands inhibit VEGF-mediated vasculogenic mimicry of prostate cancer through the AKT signaling pathway. Mol Med Rep 10:276–282. https://doi.org/10.3892/mmr.2014.2198
Burgermeister E, Seger R (2008) PPARgamma and MEK interactions in cancer. PPAR Res 2008:309469. https://doi.org/10.1155/2008/309469
Li M, Lee TW, Mok TSK et al (2005) Activation of peroxisome proliferator-activated receptor-gamma by troglitazone (TGZ) inhibits human lung cell growth. J Cell Biochem 96:760–774. https://doi.org/10.1002/jcb.20474
Vella V, Nicolosi ML, Giuliano S et al (2017) PPAR-γ agonists as antineoplastic agents in cancers with dysregulated IGF axis. Front Endocrinol (Lausanne) 8:31. https://doi.org/10.3389/fendo.2017.00031
Acknowledgements
The project described above was supported by RO1CA205632 (ATR), the Nina Hyde Center Breast Cancer Fund (ATR), T32 Training Grant in Tumor Biology CA009686 (William Kietzman), and P30CA051008 (PI: Weiner) from the National Cancer Institute for usage of the following shared resources: microscopy and imaging, tissue culture, flow cytometry, histopathology, and animal models. We thank Maria Idalia Cruz and Carlos Benitez for their technical assistance with the in vivo drug treatment. We thank Sahar Alothman and Alqassem Abuarqoub for their help with the image quantitation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have a financial relationship with Daiichi Sankyo pharmaceutical company and declare that they have no conflict of interest.
Ethical standards
The experiments in this study comply with the current laws of the country in which they were performed. The data sets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ory, V., Kietzman, W.B., Boeckelman, J. et al. The PPARγ agonist efatutazone delays invasive progression and induces differentiation of ductal carcinoma in situ. Breast Cancer Res Treat 169, 47–57 (2018). https://doi.org/10.1007/s10549-017-4649-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4649-y