Abstract
Purpose
The tumor microenvironment plays pivotal roles in promotion of many malignancies. Cancer-associated fibroblasts (CAFs) have been well-known to promote proliferation, angiogenesis, and metastasis but mechanistic understanding of tumor–stroma interactions is not yet complete. Recently, estrogen synthetic enzymes were reported to be upregulated by co-culture with stromal cells in ER positive breast carcinoma (BC) but effects of co-culture on androgen metabolism have not been extensively examined. Therefore, we evaluated roles of CAFs on androgen metabolism in ER-negative AR-positive BC through co-culture with CAFs.
Methods
Concentrations of steroid hormone in supernatant of co-culture of MDA-MB-453 and primary CAFs were measured using GC–MS. Cytokines derived from CAFs were determined using Cytokine Array. Expressions of androgen synthetic enzymes were confirmed using RT-PCR and Western blotting. Correlations between CAFs and androgen synthetic enzymes were analyzed using triple-negative BC (TNBC) patient tissues by immunohistochemistry.
Results
CAFs were demonstrated to increase expressions and activities of 17βHSD2, 17βHSD5, and 5α-Reductase1. IL-6 and HGF that were selected as potential paracrine mediators using cytokine array induced 17βHSD2, 17βHSD5, and 5α-Reductase1 expression. Underlying mechanisms of IL-6 paracrine regulation of 17βHSD2 and 17βHSD5 could be partially dependent on phosphorylated STAT3, while phosphorylated ERK could be involved in HGF-mediated 5α-Reductase1 induction. α-SMA status was also demonstrated to be significantly correlated with 17βHSD2 and 17βHSD5 status in TNBC tissues, especially AR-positive cases.
Conclusions
Results of our present study suggest that both IL-6 and HGF derived from CAFs could contribute to the intratumoral androgen metabolism in ER-negative BC patients.







Similar content being viewed by others
Abbreviations
- CAFs:
-
Cancer-associated fibroblasts
- ER:
-
Estrogen receptor
- AR:
-
Androgen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth factor receptor
- GC–MS:
-
Gas chromatography–mass spectrometry
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- TNBC:
-
Triple-negative breast carcinoma
- 17βHSD:
-
17β hydroxysteroid dehydrogenase type
- 5α-Red1:
-
5α-Reductase1
- IL-6 (-8):
-
Interleukin-6 (-8)
- HGF:
-
Hepatocyte growth factor,
- STAT3:
-
Signal transducer and activator of transcription 3
- ERK:
-
Extracellular signal-regulated kinase
- IDC:
-
Invasive ductal carcinoma
- α-SMA:
-
Α-smooth muscle actin
- FBS:
-
Fetal bovine serum
- PAI-1:
-
Plasminogen activator inhibitor-1
- MCP-1:
-
Monocyte chemotactic protein-1
- CM:
-
Conditioned medium
- siRNA:
-
Small interfering RNA
- DHT:
-
Dihydrotestosterone
- FASN:
-
Fatty acid synthase
- PRLR:
-
Prolactin receptor
References
Orimo A, Gupta P, Sgroi D, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey V, Richardson A, Weinberg R (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121(3):335–348. doi:10.1016/j.cell.2005.02.034X
Dai T, Li Z, Bai Y, Yang X, Liu J, Zhan B Shi (2015) Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res 5(10):2929–2943
Dagouassat M, Suffee N, Hlawaty H, Haddad O, Charni F, Laguillier C, Vassy R, Martin L, Schischmanoff P, Gattegno L, Oudar O, Sutton A, Charnaux N (2010) Monocyte chemoattractant protein-1 (MCP-1)/CCL2 secreted by hepatic myofibroblasts promotes migration and invasion of human hepatoma cells. Int J Cancer 126(5):1095–1108. doi:10.1002/ijc.24800
Xing F, Saidou J, Watabe K (2010) Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci 15(2):166–179. doi:10.2741/3613
Tsuyada A, Chow A, Wu J, Somlo G, Chu P, Loera S, Luu T, Li A, Wu X, Ye W, Chen S, Zhou W, Yu Y, Wang Y, Ren X, Li H, Scherle P, Kuroki Y, Wang S (2012) CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res 72(11):2768–2779. doi:10.1158/0008-5472.CAN-11-3567
Brennen W, Isaacs J, Denmeade S (2012) Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther 11(2):257–266. doi:10.1158/1535-7163.MCT-11-0340
Sund M, Kalluri R (2009) Tumor stroma derived biomarkers in cancer. Cancer Metast Rev 28(1–2):177–183. doi:10.1007/s10555-008-9175-2
Tyan S, Kuo W, Huang C, Pan C, Shew J, Chang K, Lee E, Lee W (2011) Breast cancer cells induce cancer-associated fibroblasts to secrete hepatocyte growth factor to enhance breast tumorigenesis. PLoS ONE 6(1):e15313. doi:10.1371/journal.pone.0015313
Madar S, Goldstein I, Rotter V (2013) ‘Cancer associated fibroblasts’—more than meets the eye. Trends Mol Med 19(8):447–453. doi:10.1016/j.molmed.2013.05.004
Wang X, Sang X, Diorio C, Lin S, Doillon C (2015) In vitro interactions between mammary fibroblasts (Hs 578Bst) and cancer epithelial cells (MCF-7) modulate aromatase, steroid sulfatase and 17β-hydroxysteroid dehydrogenases. Mol Cell Endocrinol 412:339–348. doi:10.1016/j.mce.2015.05.032
McNamara K, Yoda T, Miki Y, Chanplakorn N, Wongwaisayawan S, Incharoen P, Kongdan Y, Wang L, Takagi K, Takagi M, Nakamura Y, Suzuki T, Nemoto N, Miyashita M, Tamaki K, Ishida T, Ohuchi N, Sasano H (2013) Androgenic pathway in triple negative invasive ductal tumors: Its correlation with tumor cell proliferation. Cancer Sci 104(5):639–646. doi:10.1111/cas.12121
Yamaguchi Y, Takei H, Suemasu K, Kobayashi Y, Kurosumi M, Harada N, Hayashi S (2005) Tumor-stromal interaction through the estrogen-signaling pathway in human breast cancer. 65(11):4653–4662. doi:10.1158/0008-5472.CAN-04-3236
Miki Y, Suzuki T, Abe K, Suzuki S, Niikawa H, Iida S, Hata S, Akahira J, Mori K, Evans D, Kondo T, Yamada-Okabe H, Sasano H (2010) Intratumoral localization of aromatase and interaction between stromal and parenchymal cells in the non-small cell lung carcinoma microenvironment. Cancer Res 70(16):6659–6669. doi:10.1158/0008-5472.CAN-09-4653
Lehmann B, Bauer J, Chen X, Sanders M, Chakravarthy A, Shyr Y, Pietenpol J (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121(7):2750–2767. doi:10.1172/JCI45014DS1
Moore N, Buchanan G, Harris J, Selth L, Bianco-Miotto T, Hanson A, Birrell S, Butler L, Hickey T, Tilley W (2012) An androgen receptor mutation in the MDA-MB-453 cell line model of molecular apocrine breast cancer compromises receptor activity. Endocr-Related Cancer. 19(4):599–613. doi:10.1530/ERC-12-0065
Ha YW, Moon JY, Jung HJ, Chung BC, Choi MH (2009) Evaluation of plasma enzyme activities using gas chromatography-mass spectrometry based steroid signatures. J Chromatogr B 877:4125–4132
Catteau X, Simon P, Vanhaeverbeek M, Noël J (2013) Variable stromal periductular expression of CD34 and smooth muscle actin (SMA) in intraductal carcinoma of the breast. PLoS ONE 8(3):1–5. doi:10.1371/journal.pone.0057773
Chauhan H, Abraham A, Phillips J, Pringle J, Walker R, Jones J (2003) There is more than one kind of myofibroblast: analysis of CD34 expression in benign, in situ, and invasive breast lesions. J Clin Pathol 56(4):271–276. doi:10.1136/jcp.56.4.271
Suzuki T, Miki Y, Nakamura Y, Moriya T, Ito K, Ohuchi N, Sasano H (2005) Sex steroid-producing enzymes in human breast cancer. Endocr-Relate Cancer 12(4):701–720. doi:10.1677/erc.1.00834
Cochrane D, Bernales S, Jacoben B, Cittelly D, Howe E, D’Amato N, Spoelstra N, Edgerton S, Jean A, Guerrero J, Gómez F, Medicherla S, Alfaro I, McCullagh E, Jedlicka P, Torkko K, Thor A, Elias A, Protter A, Richer J (2014) Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res 16(1):R7. doi:10.1186/bcr3599
Kasasa S, Soory M (1998) The combined effects of TGF-beta, IGF and PDGF on 5alpha-reductase activity on androgen substrates in human gingival tissue. Inflammopharmacology 6(3):223–234. doi:10.1007/s10787-998-0021-5
Seo Y, Zhu B, Jeon T, Osborne T (2009) Regulation of steroid 5-alpha reductase type 2 (Srd5a2) by sterol regulatory element binding proteins and statin. Exp Cell Res 315(18):3133–3139. doi:10.1016/j.yexcr.2009.05.025
Chun J, Nadiminty N, Dutt S, Lou W, Yang J, Kung H, Evans C, Gao A (2009) Interleukin-6 regulates androgen synthesis in prostate cancer cells. Hum Cancer Biol 15(15):4815–4822. doi:10.1158/1078-0432.CCR-09-0640
Rui C, Li C, Xu W, Zhan Y, Li Y, Yang X (2008) Involvement of Egr-1 in HGF-induced elevation of the human 5alpha-R1 gene in human hepatocellular carcinoma cells. Biochem J 411(2):379–386. doi:10.1042/BJ20071343
Simard J, Gingras S (2001) Crucial role of cytokines in sex steroid formation in normal and tumoral tissues. Mol Cell Endcrinol 171(1–2):25–40. doi:10.1016/S0303-7207(00)00387-7
Grotegut S, Schweinitz D, Christofori G, Lehembre F (2006) Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J 25(15):3534–3545. doi:10.1038/sj.emboj.7601213
Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, Yoder S, Enkemann S, Eschrich S, Lee J, Beam C, Cheng J, Minton S, Muro-Cacho C, Jove R (2006) Persistent activation of Stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res 12(1):11–19. doi:10.1158/1078-0432.CCR-04-1752
Burke W, Jin X, Lin H, Huang M, Liu R, Reynolds R, Lin J (2001) Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene 20(55):7925–7934. doi:10.1038/sj.onc.1204990
Deng X, Wang S, Deng A, Liu B, Edgerton S, Lind S, Wahdan-Alaswad W, Thor A (2012) Combined effect of cyclin D3 expression and abrogation of cyclin D1 prevent mouse skin tumor development. Cell Cycle 11(2):335–342. doi:10.4161/cc.11.2.18813
Bartholomeusz C, Gonzalez-angulo A, Liu P, Hayashi N, Lluch A, Ferrer-lozano J, Hortobagyi G (2012) High ERK protein expression levels correlate with shorter survival in triple-negative breast cancer patients. Oncologist 17(6):766–774. doi:10.1634/theoncologist.2011-0377
Eralp Y, Derin D, Ozluk Y, Yavuz E, Guney N, Saip P, Muslumanoglu M, Igci A, Kücücük S, Dincer M, Aydiner A, Topuz E (2008) MAPK overexpression is associated with anthracycline resistance and increased risk for recurrence in patients with triple-negative breast cancer. Ann Oncol 19(4):669–674. doi:10.1093/annonc/mdm522
Kilvaer T, Khanehkenari M, Hellevik T, Al-Saad S, Paulsen E, Bremnes R, Busund L, Donnem T, Martinez I (2015) Cancer associated fibroblasts in stage I-IIIA NSCLC: prognostic impact and their correlations with tumor molecular markers. PLoS ONE 10(8):e0134965. doi:10.1371/journal.pone.0134965
McNamara K, Sasano H (2015) The intracrinology of breast cancer. J Steroid Biochem Mol Biol 145:172–178. doi:10.1016/j.jsbmb.2014.04.004
McNamara K, Yoda T, Nurani A, Shibahara Y, Miki Y, Wang L, Nakamura Y, Suzuki K, Yang Y, Abe E, Hirakawa H, Suzuki T, Nemoto N, Miyashita M, Tamaki K, Ishida T, Brown K, Ohuchi N, Sasano H (2014) Androgenic pathways in the progression of triple-negative breast carcinoma: a comparison between aggressive and non-aggressive subtypes. Breast Cancer Res Tr 145(2):281–293. doi:10.1007/s10549-014-2942-6
Takagi K, Miki Y, Nakagawa S, Hirakawa H, Onodera Y, Akahira J, Ishida T, Watanabe M, Kimizima I, Hayashi S, Sasano H, Suzuki T (2010) Increased intratumoral androgens in human breast carcinoma following aromatase inhibitor exemestane treatment. Endocr-Relat Cancer 17(2):415–430. doi:10.1677/ERC-09-0257
McNamara K, Yoda T, Miki Y, Nakamura Y, Suzuki T, Nemoto N, Miyashita M, Nishimura R, Arima N, Tamaki K, Ishida T, Ohuchi N, Sasano H (2015) Androgen receptor and enzymes in lymph node metastasis and cancer reoccurrence in triple-negative breast cancer. Int J Biol Markers 30(2):e184–e189. doi:10.5301/jbm.5000132
Wang C, Pan B, Zhu H, Zhou Y, Mao F, Lin Y, Xu Q, Sun Q (2016) Prognostic value of androgen receptor in triple negative breast cancer: A meta-analysis. Oncotarget 7(29):46482–46491. doi:10.18632/oncotarget.10208
Vihko P, Herrala A, Härkönen P, Isomaa V, Kaija H, Kurkela R, Li Y, Patrikainen L, Pulkka A, Soronen P, Törn S (2005) Enzymes as modulators in malignant transformation. J Steroid Biochem Mol Biol 93(2):277–283. doi:10.1016/j.jsbmb.2005.01.002
Wiebe J, Muzia D, Hu J, Szwajcer D, Hill S, Seachrist J (2000) The 4-pregnene and 5alpha-pregnane progesterone metabolites formed in nontumorous and tumorous breast tissue have opposite effects on breast cell proliferation and adhesion. Cancer Res 60(4):936–943
Narayanan R, Dalton J (2016) Androgen receptor: a complex therapeutic target for breast cancer. Cancers 8(12):108. doi:10.3390/cancers8120108
Gucalp A, Traina T (2016) Targeting the androgen receptor in triple-negative breast cancer. Curr Prob Cancer 40(2–4):141–150. doi:10.1016/j.currproblcancer.2016.09.004
Gucalp A, Tolaney S, Isakoff S, Ingle J, Liu M, Carey L, Blackwell K, Rugo H, Nabell L, Forero A, Stearns V, Doane A, Danso M, Moynahan M, Momen L, Gonzalez J, Akhtar A, Giri D, Patil S, Feigin K, Hudis C, Traina T (2013) Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res 19(19):5505–5512. doi:10.1158/1078-0432.CCR-12-3327
Barton V, D’Amato N, Gordon M, Lind H, Spoelstra N, Babbs B, Heinz R, Elias A, Jedlicka P, Jacobsen B, Richer J (2015) Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide In Vivo. Mol Cancer Ther 14(3):769–778. doi:10.1158/1535-7163.MCT-14-0926
Thakkar A, Wang B, Picon-Ruiz M, Buchwald P, Ince Tan A (2016) Vitamin D and androgen receptor-targeted therapy for triple-negative breast cancer. Breast Cancer Res Tr 157(1):77–90. doi:10.1007/s10549-016-3807-y
Narayanan R, Ahn S, Cheney M, Yepuru M, Miller D, Steiner M, Dalton J (2014) Selective androgen receptor modulators (SARMs) negatively regulate triple-negative breast cancer growth and epithelial: mesenchymal stem cell signaling. PLoS ONE 9(7):e103202. doi:10.1371/journal.pone.0103202
Chen T, Wang L, Farrar W (2000) Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res 60(23):2132–2135
Ueda T, Mawji N, Bruchovsky N, Sadar M (2002) Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J Biol Chem 277(41):38087–38094. doi:10.1074/jbc.M203313200
Ueda T, Bruchovsky N, Sadar M (2002) Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J Biol Chem 277(9):7076–7085. doi:10.1074/jbc.M108255200
Yoda T, McNamara K, Miki Y, Takagi M, Rai Y, Ohi Y, Sagara Y, Tamaki K, Hirakawa H, Ishida T, Suzuki T, Ohuchi N, Sasano H (2014) Intratumoral androgen metabolism and actions in invasive lobular carcinoma of the breast. Cancer Sci 105(11):1503–1509. doi:10.1111/cas.12535
Acknowledgements
Research for this article was supported in part by JSPS KAKENHI Grant Number 15K18396. We would like to acknowledge all the members of their laboratories, whose informal input was extremely valuable. We would also like to acknowledge the support and assistance of the members of the Department of Pathology, Tohoku University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by Ethics Committee at Tohoku University School of Medicine. Informed consent was obtained from all patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (PDF 79 kb)
Supplementary Fig. 1 CAFs induced ER negative AR positive breast carcinoma cell lines proliferation, migration, and invasion. (A) Carcinoma cell lines (MDA-MB-453, MFM-223, and SUM-185-PE) were incubated with conditioned medium (CM) derived from CAFs (TH-BC24 N and TH-BC26 N) or carcinoma cells or non-CM as negative control for 96 h. Changes of cell viability were evaluated using WST-7 assay. (B) MFM-223 cells were seeded migration or invasion chamber while CAFs were seeded in 24 well plate. After 24 h, MFM-223 cells were cultured in the absence or presence of CAFs for 24 h. The migrated or invaded cells were fixed and stained with hematoxylin. Migration or invasion rate were evaluated as the average number of cells three fields (× 200) randomly selected on the lower surface on the membrane. *, p < 0.05
10549_2017_4464_MOESM3_ESM.pdf
Supplementary material 3 (PDF 138 kb)
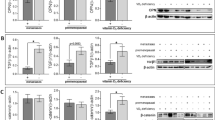
Supplementary Fig. 2 Androgen synthetic enzymes were regulated by co-culture with CAFs in ER negative breast carcinoma cells. (A) 5α-Reductase type (5α-Red) 1 and 5α-Red2 expression levels in ER negative breast cancer cell lines (MDA-MB-453, MFM-223, and SUM-185-PE) were determined using RT-PCR. (B) RT-PCR for androgen responsive genes fatty acid synthase (FASN) and prolactin receptor (PRLR) was performed from mRNA harvested from MDA-MB-453 and MFM-223 treated with control, 10 nM dihydrotestosterone (DHT), and 10 nM DHT + androgen receptor inhibitor (Bic; Bicalutamide) 10 μM for 72 h. Worth noting is the potential for a known AR mutation to alter the androgen responsiveness of the MDA-MB-453 and MFM-223 cell lines to DHT [15] although if this is the case was not assessed in this study. (C) AR mRNA expressions in ER negative breast carcinoma cells after co-culture with CAFs were determined using RT-PCR. (D) Aromatase mRNA expression in MDA-MB-453 after co-culture with CAFs was examined using RT-PCR. *, p < 0.05
Supplementary material 4 (PDF 3412 kb)
Supplementary Fig. 3 Secretions of various cytokines derived from CAFs were determined using cytokine array. (A) Cytokines were secreted from two CAFs to their conditioned medium (CM), TH-BC24 N (upper) and TH-BC26 N (bottom). Membrane images were detected using ChemiDoc XRS + System. (B) Quantification of cytokines secreted from CAFs. Membrane images were quantified using mage Lab version 5.0. (C) mRNA levels of androgen synthetic enzymes in ER negative breast carcinomas after 72 h PAI-1, MCP-1, or IL-8 treatment were determined using RT-PCR. (D) MDA-MB-453 was incubated with IL-6 or HGF with or without testosterone and androgen receptor inhibitor (bicaltamide) in serum-free medium for 72 h. Changes of cell viability were evaluated using WST-7 assay. NC; negative control, T; testosterone, Bic; bicaltamide, *, p < 0.05
Supplementary material 5 (PDF 51 kb)
Supplementary Fig. 4 Androgen synthetic enzymes were upregulated by androgen treatment in ER negative breast carcinoma cells. RT-PCR for androgen synthetic enzymes was performed from mRNA harvested from MDA-MB-453 treated with control, 10 nM dihydrotestosterone (DHT), and 10 nM DHT + androgen receptor inhibitor (Bic; bicalutamide) 10 μM for 72 h. *, p < 0.05
Rights and permissions
About this article
Cite this article
Kikuchi, K., McNamara, K.M., Miki, Y. et al. Effects of cytokines derived from cancer-associated fibroblasts on androgen synthetic enzymes in estrogen receptor-negative breast carcinoma. Breast Cancer Res Treat 166, 709–723 (2017). https://doi.org/10.1007/s10549-017-4464-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4464-5