Abstract
Purpose
While the estrogen receptor (ER) is the single most widely used biomarker to evaluate breast cancer outcomes, aspects of ER marker biology remain poorly understood. We sought to determine whether quantitative measures of ER, such as protein expression and intensity, were associated with survival, or with survival disparities experienced by Hispanic women.
Methods
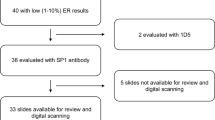
A case-cohort study included a 15% random sample of invasive breast cancer cases diagnosed from 1997 to 2009 in six New Mexico counties and all deaths due to breast cancer-related causes. Pathology reports and tissue microarrays served as sources of ER information. Analyses were restricted to women with ≥1% ER immunohistochemical staining. Hazard ratios (HR) and 95% confidence intervals (CI) for breast cancer death were estimated using Cox proportional hazards models.
Results
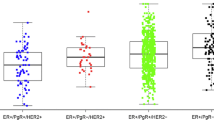
Included women represented 4336 ER+ breast cancer cases and 448 deaths. Median follow-up was 93 months. ER percent expression was not associated with breast cancer survival after adjustment for standard prognostic factors (p trend = 0.76). ER intensity remained a strong and independent risk factor for breast cancer survival in multivariate analyses: Women whose tumors expressed ER at intensity = 2 (HR 0.6; 95% CI 0.4–1.0) or 3 (HR 0.5; 95% CI 0.2–0.9) had a reduced risk of breast cancer mortality, compared to ER intensity = 1 (p trend = 0.02). Neither ER protein expression nor intensity influenced Hispanic survival disparities.
Conclusions
Estrogen receptor percent positive staining is not independently related to breast cancer survival after adjustment for other survival-related factors. ER intensity, in contrast, demonstrates promise for prognostic utility.


Similar content being viewed by others
References
Knight WA, Livingston RB, Gregory EJ, McGuire WL (1977) Estrogen receptor as an independent prognostic factor for early recurrence in breast cancer. Can Res 37(12):4669–4671
Walt AJ, Singhakowinta A, Brooks SC, Cortez A (1976) The surgical implications of estrophile protein estimations in carcinoma of the breast. Surgery 80(4):506–512
Early Breast Cancer Trialists’ Collaborative, Davies C, Godwin J, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–784. doi:10.1016/S0140-6736(11)60993-8
Viale G, Regan MM, Maiorano E, Mastropasqua MG, Dell’Orto P, Rasmussen BB, Raffoul J, Neven P, Orosz Z, Braye S, Ohlschlegel C, Thurlimann B, Gelber RD, Castiglione-Gertsch M, Price KN, Goldhirsch A, Gusterson BA, Coates AS (2007) Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 25(25):3846–3852. doi:10.1200/JCO.2007.11.9453
Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C, Cuzick J, Houghton J, Williams N, Mallon E, Bishop H, Ellis I, Larsimont D, Sasano H, Carder P, Cussac AL, Knox F, Speirs V, Forbes J, Buzdar A (2008) Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol 26(7):1059–1065. doi:10.1200/JCO.2007.12.9437
Elledge RM, Green S, Pugh R, Allred DC, Clark GM, Hill J, Ravdin P, Martino S, Osborne CK (2000) Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int J Cancer 89(2):111–117
McGuire WL (1978) Steroid receptors in human breast cancer. Can Res 38(11 Pt 2):4289–4291
Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clini Oncol 17(5):1474–1481. doi:10.1200/jco.1999.17.5.1474
Ferno M, Stal O, Baldetorp B, Hatschek T, Kallstrom AC, Malmstrom P, Nordenskjold B, Ryden S (2000) Results of two or five years of adjuvant tamoxifen correlated to steroid receptor and S-phase levels. South Sweden Breast Cancer Group, and South-East Sweden Breast Cancer Group. Breast Cancer Res Treat 59(1):69–76
Anderson H, Hills M, Zabaglo L, A’Hern R, Leary AF, Haynes BP, Smith IE, Dowsett M (2011) Relationship between estrogen receptor, progesterone receptor, HER-2 and Ki67 expression and efficacy of aromatase inhibitors in advanced breast cancer. Ann Oncol 22(8):1770–1776. doi:10.1093/annonc/mdq700
Kinsel LB, Szabo E, Greene GL, Konrath J, Leight GS, McCarty KS Jr (1989) Immunocytochemical analysis of estrogen receptors as a predictor of prognosis in breast cancer patients: comparison with quantitative biochemical methods. Can Res 49(4):1052–1056
Ma H, Lu Y, Marchbanks PA, Folger SG, Strom BL, McDonald JA, Simon MS, Weiss LK, Malone KE, Burkman RT, Sullivan-Halley J, Deapen DM, Press MF, Bernstein L (2013) Quantitative measures of estrogen receptor expression in relation to breast cancer-specific mortality risk among white women and black women. Breast Cancer Res 15(5):R90. doi:10.1186/bcr3486
O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, Dressler LG, Geradts J, Millikan RC (2010) Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res 16(24):6100–6110. doi:10.1158/1078-0432.CCR-10-1533
Ma H, Lu Y, Malone KE, Marchbanks PA, Deapen DM, Spirtas R, Burkman RT, Strom BL, McDonald JA, Folger SG, Simon MS, Sullivan-Halley J, Press MF, Bernstein L (2013) Mortality risk of black women and white women with invasive breast cancer by hormone receptors, HER2, and p53 status. BMC cancer 13:225. doi:10.1186/1471-2407-13-225
Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, Perez EA, Saphner T, Wolff AC, Sledge GW Jr, Wood WC, Davidson NE (2012) Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst 104(5):406–414
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF, American Society of Clinical Oncology/College of American P (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 131(1):18–43. doi:10.1043/1543-2165(2007)131[18:ASOCCO]2.0.CO;2
Barlow WE (1994) Robust variance estimation for the case-cohort design. Biometrics 50(4):1064–1072
Xue X, Xie X, Gunter M, Rohan TE, Wassertheil-Smoller S, Ho GY, Cirillo D, Yu H, Strickler HD (2013) Testing the proportional hazards assumption in case-cohort analysis. BMC Med Res Methodol 13:88. doi:10.1186/1471-2288-13-88
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of the NCIEWGoCD (2005) Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 97(16):1180–1184. doi:10.1093/jnci/dji237
Chung GG, Zerkowski MP, Ghosh S, Camp RL, Rimm DL (2007) Quantitative analysis of estrogen receptor heterogeneity in breast cancer. Lab Investig 87(7):662–669. doi:10.1038/labinvest.3700543
Hammond ME, Hayes DF, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC, American Society of Clinical O, College of American P (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134(7):e48–e72
National Cancer Institute D (2016) Surveillance Research Program, Surveillance Systems Branch. Surveillance, Epidemiology, and End Results (SEER) Program Research Data (1973–2013). www.seer.cancer.gov
Jacobs TW, Prioleau JE, Stillman IE, Schnitt SJ (1996) Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst 88(15):1054–1059
Combs SE, Han G, Mani N, Beruti S, Nerenberg M, Rimm DL (2016) Loss of antigenicity with tissue age in breast cancer. Lab Investig 96(3):264–269. doi:10.1038/labinvest.2015.138
Fisher B, Redmond C, Fisher ER, Caplan R (1988) Relative worth of estrogen or progesterone receptor and pathologic characteristics of differentiation as indicators of prognosis in node negative breast cancer patients: findings from National Surgical Adjuvant Breast and Bowel Project Protocol B-06. J clin oncol 6(7):1076–1087. doi:10.1200/JCO.1988.6.7.1076
Barnes DM, Harris WH, Smith P, Millis RR, Rubens RD (1996) Immunohistochemical determination of oestrogen receptor: comparison of different methods of assessment of staining and correlation with clinical outcome of breast cancer patients. Br J Cancer 74(9):1445–1451
Lockwood CA, Ricciardelli C, Raymond WA, Seshadri R, McCaul K, Horsfall DJ (1999) A simple index using video image analysis to predict disease outcome in primary breast cancer. Int J Cancer 84(3):203–208
Alexieva-Figusch J, Van Putten WL, Blankenstein MA, Blonk-Van Der Wijst J (1988) The prognostic value and relationships of patient characteristics, estrogen and progestin receptors, and site of relapse in primary breast cancer. Cancer 61(4):758–768
McCarty KS Jr, Miller LS, Cox EB, Konrath J, McCarty KS Sr (1985) Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 109(8):716–721
McCarty KS Jr, Szabo E, Flowers JL, Cox EB, Leight GS, Miller L, Konarth J, Soper JT, Budwit DA, Creasman WT (1986) Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Can Res 46(8 Suppl):4244s–4248s
Thorpe SM, Christensen IJ, Rasmussen BB, Rose C (1993) Short recurrence-free survival associated with high oestrogen receptor levels in the natural history of postmenopausal, primary breast cancer. Eur J Cancer 29A(7):971–977
Black R, Prescott R, Bers K, Hawkins A, Stewart H, Forrest P (1983) Tumour cellularity, oestrogen receptors and prognosis in breast cancer. Clin Oncol 9(4):311–318
Sancho-Garnier H, Delarue JC, Mouriesse H, Contesso G, May-Levin F, Gotteland M, May E (1995) Is the negative prognostic value of high oestrogen receptor (ER) levels in postmenopausal breast cancer patients due to a modified ER gene product? Eur J Cancer 31A(11):1851–1855
Fowler AM, Solodin N, Preisler-Mashek MT, Zhang P, Lee AV, Alarid ET (2004) Increases in estrogen receptor-alpha concentration in breast cancer cells promote serine 118/104/106-independent AF-1 transactivation and growth in the absence of estrogen. FASEB J 18(1):81–93. doi:10.1096/fj.03-0038com
Acknowledgements
This study was supported by Grants R01CA 132877 and 2P30CA11810 from the National Cancer Institute (NCI), and NCI contract HSN26120130010I-Task Order HHSN26100005 to the Surveillance Epidemiology End Results (SEER) program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hill, D.A., Barry, M., Wiggins, C. et al. Estrogen receptor quantitative measures and breast cancer survival. Breast Cancer Res Treat 166, 855–864 (2017). https://doi.org/10.1007/s10549-017-4439-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4439-6