Abstract
Purpose
The increase in clinical trials with androgen receptor (AR)-targeting drugs emphasizes the need of clarifying the role of AR expression in different breast cancer subtypes. AR confers good prognosis in estrogen receptor positive (ER+) breast cancer, but its role in ER-negative (ER−) breast cancer is unclear. The aim of this study was to elaborate on previous findings of a differential prognostic role for AR depending on ER status, using breast cancer mortality (BCM) as endpoint, in a population-based cohort from the Malmö Diet and Cancer Study.
Methods
Immunohistochemical AR expression was assessed in 910 women with invasive breast cancer diagnosed 1991–2010, supplemented with clinicopathological information, vital status, and cause of death, with the last follow-up in December 2014 (median 10 years). Survival analyses according to AR status and AR/ER combinations were performed.
Results
AR expression was available for 671 tumors. AR+ (n = 573, 85%) was associated with favorable established tumor markers and lower BCM in univariable analysis, especially during the first 5 years following diagnosis [HR 0.4; 95% confidence intervals (CI) 0.2–0.7]. Multivariable analysis for short-term follow-up indicated higher BCM among patients with AR+ER− tumors (HR 3.5; 95% CI 1.4–9.1) than other AR and ER combinations.
Conclusions
AR expression added prognostic information to ER expression with respect to short-term prognosis. The worst prognosis was seen for patients with AR+/ER− tumors in short-term follow-up, supporting the pre-specified hypothesis. However, larger cohorts are needed for further characterization of the role of AR expression in ER− breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The heterogeneous nature of breast cancer highlights the importance of identifying prognostic and predictive markers for clinical implementation of tailored treatment. Recently, there has been increasing interest in the androgen receptor (AR) as a potential prognostic biomarker and treatment target [1, 2]. The prognostic role of AR has consistently been described as favorable for breast cancer in general and within the estrogen receptor-α positive (ER+) subgroup [3]. Some studies have reported that AR is prognostically beneficial irrespective of ER [4], but the results regarding the role of AR expression in the ER negative (ER−) setting diverge [2, 4,5,6,7,8]. Ongoing clinical trials are evaluating anti-androgens and selective androgen receptor modulators across different breast cancer subtypes [2, 9, 10]. It is essential to better define AR’s prognostic role in different breast cancer subtypes.
The most recent meta-analysis reported AR to be prognostically favorable irrespective of ER expression, but only included studies published until early 2015 [11]. In late 2015, we reported a differential role of AR depending on the ER status of the tumor using disease-free survival (DFS) as endpoint in a large population-based observational cohort. Patients with AR-positive (AR+) ER+ tumors had superior prognosis compared to all other AR/ER combinations. Markedly, the patients with AR+ER− tumors had worse prognosis in all adjusted models compared to patients with AR-negative (AR−) ER− tumors, and there was significant interaction between AR and ER expression with respect to DFS [8].
The aim of this study was to validate and elaborate the previous findings of a differential role for AR on the outcome depending on ER status, using breast cancer-related death as endpoint, in an independent cohort with longer follow-up, the population-based Malmö Diet and Cancer Study (MDCS). We hypothesized that AR positivity would be associated with overall favorable tumor characteristics and prognosis and that analyses stratified by tumor ER status would reveal a differential role of AR depending on ER. The primary endpoint was breast cancer mortality (BCM), and the secondary endpoint was all-cause mortality. Another aim was to investigate whether AR could add long-term prognostic information.
Materials and methods
The Malmö Diet and Cancer Study
The population-based prospective MDCS was initiated to examine associations between diet and cancer [12] and included people living in Malmö, Sweden in 1991–1996. Swedish language skills and mental abilities sufficient to understand the questionnaire were required for enrollment. The participation rate was 40% of the source population. A previous report showed lower mortality due to cancer and lifestyle-related causes among participants during recruitment and follow-up compared to non-participants [13]. Baseline data were collected from interviews, questionnaires, and examinations.
The female MDCS cohort consists of 17,035 women (born 1923–1950). Information on incident breast cancer and vital status were annually retrieved from the Swedish Cancer Registry, the South Swedish Regional Tumor Registry, and the Swedish Cause of Death Registry. Ethical permission was obtained from the Ethical Committee at Lund University (Dnr 472/2007). All participants signed a written informed consent form.
Study population and patient characteristics
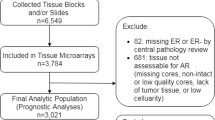
This is a case-only analysis of incident primary breast cancer within the MDCS, and patients with a previous breast cancer diagnosis at enrollment (n = 576) were therefore excluded (Fig. 1). During follow-up until December 31, 2010, a total of 1016 women were identified with incident breast cancer through record linkage. Patient characteristics at diagnosis were obtained from medical records. To investigate invasive tumor characteristics in relation to survival, in situ only cancers (n = 68) and patients who received neo-adjuvant treatments (n = 4) were excluded. Patients were also excluded if they had distant metastasis at diagnosis (n = 14) or died from breast cancer-related causes ≤0.3 years from diagnosis (n = 2). Patients with bilateral cancers (n = 17) were excluded due to difficulties in evaluating the relation between tumor characteristics and prognosis. Finally, one patient who declined treatment for four years before accepting surgery was excluded. Thus, the final study population consisted of 910 patients. Information on cause of death and vital status was retrieved from the Swedish Causes of Death Registry, with last follow-up December 31st, 2014.
Tumor and histopathological analyses
Information on tumor size, grade, and axillary lymph node involvement (ALNI) was retrieved from pathology reports for tumors from 2005 and onwards. From 2008 onwards, information was also obtained for the immunohistochemistry (IHC) based markers ER, progesterone receptor (PR), proliferation index (Ki67), and human epidermal growth factor receptor-2 (HER2) status. From 1991 to 2007, ER, PR, Ki67 and HER2 were assessed using tissue microarrays (TMAs) [14]. A pathological re-evaluation was performed regarding invasiveness and grade for tumors diagnosed prior to 2006 [15]. A cut-off for positivity of >10% positively stained nuclei was applied for ER, PR, and Ki67. Regarding HER2, in situ hybridization (ISH) results were used when available. HER2 IHC was considered positive (HER2+) when annotated as 3+ and negative (HER2−) for 0 or 1+. HER2 IHC scorings of 2+ were categorized as missing if not confirmed to be amplified or normal in ISH analyses.
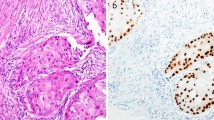
A TMA was constructed with two 1 mm cores from each tumor (Beecher, WI, USA). Among the 910 patients included in the study population, tumor tissue was available from 718 patients (Fig. 1). For IHC analysis of AR, 4-μm sections were automatically pretreated using the PT Link system and stained (Autostainer Plus, Dako, DK) for monoclonal antibody Ab-1 (clone AR441, dilution 1:200, Thermo Scientific). Microscopy assessments were performed using digital pathology (PathXL, http://www.pathxl.com). Following assessment of tumor invasiveness using hematoxylin and eosin stained slides, a semi-quantitative scoring of AR fractions (0, 1–10, 11–50, 51–75, and 76–100%) of positively stained nuclei, irrespective of nuclear staining intensity, was performed by one observer (KE), Fig. 2. If the cores from one tumor were discordant, a final score was evaluated across both cores. The AR variable was dichotomized using a cut-off of 10% as pre-specified in a statistical plan aimed at elaborating on our previous findings [8], and to avoid an exploratory study design approach. This study adheres to the REMARK criteria [16].
Statistical analyses
The distribution of patient and tumor characteristics at diagnosis in relation to AR expression was categorized and presented as percentages. Continuous variables are presented as the median and interquartile range (IQR). Distributional differences were assessed by X 2-analyses, logistic regression, or Mann–Whitney U tests as appropriate.
The association between AR and prognosis was examined with BCM as the primary endpoint. BCM was defined as the incidence of breast cancer-related death (when breast cancer was the cause of death or the contributing cause of death). The secondary endpoint was all-cause mortality, i.e., death from any cause. Follow-up was calculated from the date of breast cancer diagnosis to the date of breast cancer-related death, date of death from another cause, date of emigration or the end of follow-up as of December 31, 2014.
Firstly, AR expression in relation to BCM was assessed by comparison of cumulative BCM (CBCM) for patients with AR+ and AR− tumors in the overall population and stratified by ER status. Rather than analyzing cause-specific survival, this method [17] takes competing risks into account, which was considered relevant due to the relatively long follow-up (median 10 years) and high median age at diagnosis of the patients (64.9 years). AR in relation to all-cause mortality was assessed by analyzing cumulative all-cause mortality (CM), both overall and stratified by ER status. Differences in CBCM and CM between subgroups were evaluated by the LogRank test. AR in relation to BCM was further investigated by cause-specific Cox proportional hazards analysis with follow-up censored when a death from another cause occurred. Effects are presented as the hazard ratios (HR) and 95% confidence intervals (CI) for the overall population, stratified by ER status, and for combinations of AR and ER status using the AR+ER+ subgroup as reference.
Adjustments were made in two multivariable models including the following covariates. Model 1 included age at diagnosis (continuous), tumor size >20 mm (yes/no), ALNI (≥1 yes/no), histological grade (III vs. I–II), and ER status (±). Model 2 included the covariates from Model 1 with the addition of planned adjuvant treatments, chemotherapy (yes/no), radiotherapy (yes/no), and endocrine treatment (yes/no). An interaction term between AR and ER was added to the Cox models to evaluate the strength of evidence against the null hypothesis of no interaction between these factors on outcome. Proportional hazards assumptions were evaluated visually by inspecting the log minus log survival curves and formally using Schoenfeld’s test. There was weak evidence for a non-proportional hazards effect on AR ± for the overall follow-up (P = 0.06). The follow-up time was subsequently divided into categories of 0–5 years and >5 years, where the proportional hazards assumption was better met, and survival analyses by AR status were repeated for 0–5 years and >5 years of follow-up.
All tests were two-sided, and P values should be interpreted as the level of evidence against each null hypothesis. Nominal P values without adjustments for multiple testing are presented. The user-contributed program stcompet.ado for the statistics package Stata version 14.1 (StataCorp LP, College Station, TX, USA) was used to estimate cause-specific cumulative mortality. Stata was also used to draw the cumulative mortality graphs and to test proportional hazards assumptions. All other statistical analyses were performed in SPSS 22.0 (IBM).
Results
AR expression, patient, and tumor characteristics
Tumor AR expression was assessable in 671 of 718 cases (93%) where tumor tissue was available (Fig. 1). The distribution of AR expression included 573 AR+ tumors (85%) and 98 AR− tumors (15%). AR+ status was associated with smaller tumor size, lower histological grade, ER/PR co-expression, and low proliferation index (Ki67 ≤ 10%). Within the ER−PR-negative (PR–) subset, 34% (15/44) of HER2– tumors (triple-negative breast cancer, TNBC) were AR+, and among HER2-positive tumors, 86% (12/14) were AR+. More patients with AR− tumors had received adjuvant chemotherapy and died from breast cancer-related causes compared to patients with AR+ tumors (Table 1).
Cumulative breast cancer mortality and all-cause mortality by AR expression
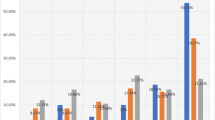
During follow-up, 178 of the patients included in survival analyses died, of whom 93 died from breast cancer-related causes (Table 1). The median follow-up period for patients who were still alive by the end of 2014 was 10 years. The incidence of breast cancer-related death was graphically illustrated by CBCM plots (Fig. 3). CBCM was lower among patients with AR+ tumors compared to patients with AR− tumors (LogRank P = 0.002). When stratified by ER status, the CBCM observed among all patients with AR+ tumors was attributable to patients with AR+ER+ rather than AR+ER− tumors. However, the evidence for an association between AR+ER+ and lower CBCM was weak (LogRank P = 0.20) compared to AR−ER+. During the first 5 years of follow-up, no difference in CBCM by AR+ was observed for patients with ER− tumors. AR+ was weakly associated with higher all-cause mortality (LogRank P = 0.08), but this association was not seen in CM analyses stratified by ER status (Fig. 4). Cox regression analyses were performed to further investigate CBCM in relation to AR status, as well as to ER status and time from diagnosis to breast cancer-related death. No significant prognostic differences depending on diagnostic period before and after the trastuzumab introduction (≤year 2005 vs. 2006+) was revealed.
Breast cancer mortality by AR and ER expression for overall follow-up
For the overall follow-up period, the incidence of BCM for patients with AR+ tumors was half that of patients with AR− tumors in univariable Cox analyses (HR 0.48: 95% CI 0.30–0.77; P = 0.002, Table 2). However, after adjustments for potential confounders, no risk reduction by AR+ remained (Table 2, models 1 and 2). When associations between AR and incidence of breast cancer-related death were assessed in the ER+ and ER− subgroups separately, no prognostic impact by AR was seen in either group. Furthermore, no interaction was observed between AR and ER (P interaction ≥ 0.58) regarding BCM.
In the analyses of AR/ER combinations, patients with AR−ER− as well as AR+ER− tumors had increased risk of CBCM compared to patients with AR+ER+ tumors. After adjustments for age and tumor characteristics (Table 2, model 1), there was slight evidence that patients with AR+ER− tumors had higher incidence of breast cancer-related death compared to patients with AR+ER+ tumors. However, this association did not remain after adjusting for adjuvant treatments.
Breast cancer mortality by AR and ER in short-term versus long-term follow-up
Separate analyses were performed for the initial 5-year period and from 5 years onwards (Table 2). This was done to better meet the assumption of proportional hazards and to differentiate between potential early and late impacts of tumor AR on BCM.
The lower BCM in the AR+ group observed in the overall period seemed driven by a lower BCM relative to AR− during the first 5 years (HR 0.37: 95% CI 0.19–0.71; P = 0.003, 43 events). The corresponding figures for the interval from five years and onwards pointed in a similar direction, although non-significant (HR 0.61: 95% CI 0.31–1.20; P = 0.15, 50 events). In the adjusted models for ER+ versus ER− strata during the short-term follow-up, the estimated hazard ratios for BCM by AR diverged to a higher degree compared to the corresponding estimate in the overall follow-up period. However, no interactions between AR and ER status were observed (P interaction ≥ 0.35). After adjustments for age and tumor characteristics (model 1, 0–5 years), there was moderate evidence that patients with AR+ER− tumors had higher BCM during the short-term follow-up compared to patients with AR+ER+ tumors. In this model, BCM for patients with AR+ER− tumors was more than threefold that of patients with AR+ER+ tumors. Furthermore, patients with AR+ER− tumors had almost double the BCM compared with patients with AR−ER− tumors. When further adjustments for adjuvant treatment were incorporated into the model (model 2), the patients with AR+ER− tumors still had worse prognosis compared to all other AR/ER combinations.
Discussion
In this prospective cohort with long-term follow-up, breast cancer patients with AR+ tumors had lower BCM but not all-cause mortality compared to patients with AR− tumors. AR+ was not an independent prognostic factor, and the hypothesis of an interaction between AR and ER was not confirmed. However, in line with our hypothesis, patients with AR+ER− tumors had the worst prognosis of all AR/ER combinations, as demonstrated in multivariable analyses with short-term follow-up.
The current interest in AR in breast cancer is multilayered. AR is a promising primary target for treatment in ER− breast cancer and especially in TNBC, where treatment options are scarce. Furthermore, specific molecular subgroups with androgen-regulated genetic signatures have emerged [18,19,20]. For example, the Luminal AR (LAR) subtype has been associated with a clearly different clinical course from that of the other TNBC subtypes [21,22,23].
In ER+ disease, AR antagonizes the proliferative effect of ER signaling [24]. AR may improve prediction of endocrine response [25, 26] and also offer an alternative endocrine treatment target when anti-estrogen regimens fail. Studies focused on integrating AR with other transcription factors such as ER are warranted to better understand the associations between AR and survival across breast cancer subtypes [2]. One possible next step would be to address AR as a ratio of ER, as done previously [22]. This may enhance the understanding of AR actions, due to the suggested competitive interactions between these two receptors [27].
We conclude that AR adds information for BCM compared to that of ER alone, but in the present study only to a limited extent. The findings of AR status on BCM did not remain after adjustments for confounders, suggesting that AR did not drive the association. Instead, ER was interpreted as the primary driver since AR and ER were highly co-expressed, and the association with BCM remained for patients with ER+ tumors in short-term adjusted analyses (data not shown). This interpretation was supported by the unadjusted analysis of combined AR/ER. However, in line with our hypothesis, AR added short-term prognostic information after adjustment for confounders. The poorest prognosis was seen among patients with AR+ER− tumors. This was also true after adjustment for treatment, but the adjusted HRs were closer to 1.0, and the evidence for higher BCM compared to the other subgroups was weaker after adjustment. If AR+ER− breast cancer is confirmed to have inferior prognosis, this may impact the choice of AR-targeted treatments and reveal a need for closer surveillance of this patient group.
There are several suggested reasons as to why we could not fully confirm our hypothesis. Our previous results on AR expression considered DFS in a cohort of median follow-up of 5 years. The time from diagnosis to breast cancer-related death may not be comparable with the event of local or regional recurrence, contralateral cancer, or distant metastasis, as evaluated previously [8]. There may also be biological differences in the AR effect in relation to these different endpoints. For the follow-up beyond 5 years, there was a selection of healthier patients with more favorable biology. Furthermore, the patients in the MDCS were diagnosed earlier (from 1991 to 2010) and during a 19-year period, with indications for treatment regimens such as tamoxifen treatment changing over time, where tamoxifen was initially prescribed according to menopausal status rather than ER status [28]. Our initial results [8] were based on the BC Blood Study, which was initiated in 2002 and included more aromatase inhibitor-treated patients.
AR+ was confirmed to be associated with favorable tumor characteristics, as consistently shown previously [8, 29, 30]. In the present study, the proportion of patients with AR+ tumors (85%) was equal to that of the independent cohort BC Blood Study (AR+ 85%), in which the same antibody was used [8]. This provides a measure of external validity to the AR assessments, since similar exclusion criteria were adopted. Both studies involved population-based Swedish cohorts, and similar distributions could thus be expected.
The strengths in this study were the long-term follow-up and a study population from a population-based, well-characterized cohort [13, 31]. Since the median age at diagnosis was greater than 60 years, the findings are mostly applicable to postmenopausal breast cancer. The completeness of the Swedish Cancer Registry is considered to be high with low underreporting of breast cancer [32]. The Swedish Cause of Death Registry has been reported to have complete and valid data from an international perspective, with the highest accuracy for cancer diagnoses [33].
A lack of standardization of AR antibodies and cut-offs used has been common and may explain some discrepancies across studies [4, 11]. In the present study, a validated antibody and a common cut-off were used [4]. The amount of missing AR values among patients for which tumor tissue was available was low and was similar in distribution to the overall population and was not considered a to exert a risk for selection bias. However, patients with missing tumor tissue were older and had smaller tumors, with a lower proliferation index and grade. They also more often had node-negative disease, suggesting under-representation of tumors with favorable characteristics in the material, which constitutes a selection bias. Thus, the true proportion of AR+ may be slightly higher than the value presented in this paper, and the incidence of BCM may be slightly lower in the underlying population from which the study population was sampled.
The tumor marker data were derived from TMAs for patients diagnosed up until 2007. Thereafter, routine clinical pathology reports were used. For the PR variable, this introduced a systematic bias due to higher positive frequency in clinical data as compared to TMA data. Thus, PR was only addressed in the descriptive analyses and should be interpreted with caution. In contrast, ER status did not depend on the diagnostic period. The missing ER was addressed by presenting the associated HRs separately.
This study indicates that cohorts with larger sample sizes are needed to further elucidate the prognostic role of AR in ER− breast cancer. Indeed, there is a need to incorporate several hormonal receptors in future studies of prognosis and treatment prediction [34]. AR may be valuable for short-term prognosis, whereas additional specific markers may be needed for long-term prognosis for ER+ breast cancer, which tends to relapse late [35].
The number of ongoing clinical studies involving AR targeting is steadily expanding and has included patients with both early and advanced breast cancer and of different subtypes [36,37,38,39] (ClinicalTrials.govidentifier: NCT01842321, NCT01889238, NCT02689427, NCT02676986, NCT02457910, NCT02463032, NCT02368691). Identification of subtypes based on gene expression analyses have provided a more comprehensive picture of hormonal regulation, such as the LAR subtype which is heavily enriched in hormonally regulated pathways in spite of being ER− [21].
There is yet no consensus on whether AR antagonists or agonists are preferable as targeted treatments in any breast cancer subtype. The forthcoming reports of ongoing clinical trials—combined with observational studies and mechanistic studies on treatment response/resistance [40,41,42,43,44]—will impact the future direction. Most likely, a wider clinical implementation of AR assessments will be of highest value for patients with TNBC [23] and for patients in the metastatic setting that have suffered from treatment resistance, and may benefit from the addition of AR-targeted treatments such as enzalutamide [22, 44,45,46].
In conclusion, AR+ was associated with lower BCM in the overall study population and for the overall and short-term follow-up intervals, but not for long-term follow-up. The poorest prognosis was seen among patients with AR+ER− tumors after adjustment for confounders in the short-term follow-up. Future studies on the role of AR in breast cancer require larger cohorts, especially in the ER− subset, and the inclusion of gene expression analyses may add valuable information.
Abbreviations
- ALNI:
-
Axillary lymph node involvement
- AR:
-
Androgen receptor
- BCM:
-
Breast cancer mortality
- CBCM:
-
Cumulative breast cancer mortality
- CI:
-
Confidence interval
- CM:
-
Cumulative all-cause mortality
- DFS:
-
Disease-free survival
- ER:
-
Estrogen receptor alpha
- HER2:
-
Human epidermal growth factor receptor -2
- HR:
-
Hazard ratio
- IHC:
-
Immunohistochemistry
- IQR:
-
Interquartile range
- ISH:
-
In situ hybridization
- Ki67:
-
Proliferation associated antigen
- LAR:
-
Luminal AR
- MDCS:
-
Malmö Diet and Cancer Study
- PR:
-
Progesterone receptor
- TMA:
-
Tissue microarray
- TNBC:
-
Triple-negative breast cancer
References
Garay JP, Park BH (2012) Androgen receptor as a targeted therapy for breast cancer. Am J Cancer Res 2(4):434–445
McNamara KM, Sasano H (2016) Androgen and breast cancer: an update. Curr Opin Endocrinol Diabetes Obes 23(3):249–256
Kim Y, Jae E, Yoon M (2015) Influence of androgen receptor expression on the survival outcomes in breast cancer: a meta-analysis. J Breast Cancer 18(2):134–142
Vera-Badillo FE, Templeton AJ, de Gouveia P, Diaz-Padilla I, Bedard PL, Al-Mubarak M, Seruga B, Tannock IF, Ocana A, Amir E (2014) Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst 106(1):djt319
McNamara KM, Moore NL, Hickey TE, Sasano H, Tilley WD (2014) Complexities of androgen receptor signalling in breast cancer. Endocr Relat Cancer 21(4):T161–T181
Jiang HS, Kuang XY, Sun WL, Xu Y, Zheng YZ, Liu YR, Lang GT, Qiao F, Hu X, Shao ZM (2016) Androgen receptor expression predicts different clinical outcomes for breast cancer patients stratified by hormone receptor status. Oncotarget 7(27):41285–41293
Choi JE, Kang SH, Lee SJ, Bae YK (2015) Androgen receptor expression predicts decreased survival in early stage triple-negative breast cancer. Ann Surg Oncol 22(1):82–89
Elebro K, Borgquist S, Simonsson M, Markkula A, Jirstrom K, Ingvar C, Rose C, Jernstrom H (2015) Combined androgen and estrogen receptor status in breast cancer: treatment prediction and prognosis in a population-based prospective cohort. Clin Cancer Res 21(16):3640–3650
Overmoyer B, Sanz-Altamira P, Taylor RP, Hancock ML, Dalton JT, Johnston MA, Steiner MS (2014) Enobosarm: a targeted therapy for metastatic, androgen receptor positive, breast cancer. ASCO Meet Abstr 32(15_suppl):568
Proverbs-Singh T, Feldman JL, Morris MJ, Autio KA, Traina TA (2015) Targeting the androgen receptor in prostate and breast cancer: several new agents in development. Endocr Relat Cancer 22(3):R87–R106
Bozovic-Spasojevic I, Zardavas D, Brohee S, Ameye L, Fumagalli D, Ades F, De Azambuja E, Bareche Y, Piccart M, Paesmans M, Sotiriou C (2016) The prognostic role of androgen receptor in patients with early stage breast cancer: a meta-analysis of clinical and gene expression data. Clin Cancer Res 22(11):2702–2712
Berglund G, Elmstahl S, Janzon L, Larsson SA (1993) The Malmo Diet and Cancer Study. design and feasibility. J Intern Med 233(1):45–51
Manjer J, Carlsson S, Elmstahl S, Gullberg B, Janzon L, Lindstrom M, Mattisson I, Berglund G (2001) The Malmo Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev 10(6):489–499
Borgquist S, Djerbi S, Ponten F, Anagnostaki L, Goldman M, Gaber A, Manjer J, Landberg G, Jirstrom K (2008) HMG-CoA reductase expression in breast cancer is associated with a less aggressive phenotype and influenced by anthropometric factors. Int J Cancer 123(5):1146–1153
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19(5):403–410
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100(2):229–235
Marubini E, Valsecchi MG (1995) Analysing survival data from clinical trials and observational studies. Wiley, New York
Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, Macgrogan G, Bergh J, Cameron D, Goldstein D, Duss S, Nicoulaz AL, Brisken C, Fiche M, Delorenzi M, Iggo R (2005) Identification of molecular apocrine breast tumours by microarray analysis. Oncogene 24(29):4660–4671
Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, Gerald WL (2006) An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 25(28):3994–4008
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Investig 121(7):2750–2767
Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD, Pietenpol JA, Hortobagyi GN, Symmans WF, Ueno NT (2013) Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res 19(19):5533–5540
Cochrane DR, Bernales S, Jacobsen BM, Cittelly DM, Howe EN, D’Amato NC, Spoelstra NS, Edgerton SM, Jean A, Guerrero J, Gomez F, Medicherla S, Alfaro IE, McCullagh E, Jedlicka P, Torkko KC, Thor AD, Elias AD, Protter AA, Richer JK (2014) Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res 16(1):R7
Barton VN, D’Amato NC, Gordon MA, Christenson JL, Elias A, Richer JK (2015) Androgen receptor biology in triple negative breast cancer: a case for classification as AR+ or quadruple negative disease. Horm Cancer 6(5–6):206–213
Pietri E, Conteduca V, Andreis D, Massa I, Melegari E, Sarti S, Cecconetto L, Schirone A, Bravaccini S, Serra P, Fedeli A, Maltoni R, Amadori D, De Giorgi U, Rocca A (2016) Androgen receptor signaling pathways as a target for breast cancer treatment. Endocr Relat Cancer 23(10):R485–R498
Park S, Park HS, Koo JS, Yang WI, Kim SI, Park BW (2012) Higher expression of androgen receptor is a significant predictor for better endocrine-responsiveness in estrogen receptor-positive breast cancers. Breast Cancer Res Treat 133(1):311–320
Hilborn E, Gacic J, Fornander T, Nordenskjold B, Stal O, Jansson A (2016) Androgen receptor expression predicts beneficial tamoxifen response in oestrogen receptor-alpha-negative breast cancer. Br J Cancer 114(3):248–255
Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, Jindal S, Segara D, Jia L, Moore NL, Henshall SM, Birrell SN, Coetzee GA, Sutherland RL, Butler LM, Tilley WD (2009) Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Can Res 69(15):6131–6140
Early Breast Cancer Trialists’ Collaborative Group (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351(9114):1451–1467
Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, Marotti JD, Hankinson SE, Colditz GA, Tamimi RM (2011) Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res 17(7):1867–1874
Park S, Koo J, Park HS, Kim JH, Choi SY, Lee JH, Park BW, Lee KS (2010) Expression of androgen receptors in primary breast cancer. Ann Oncol 21(3):488–492
Manjer J, Elmstahl S, Janzon L, Berglund G (2002) Invitation to a population-based cohort study: differences between subjects recruited using various strategies. Scand J Public Health 30(2):103–112
Barlow L, Westergren K, Holmberg L, Talback M (2009) The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol 48(1):27–33
Johansson LA, Bjorkenstam C, Westerling R (2009) Unexplained differences between hospital and mortality data indicated mistakes in death certification: an investigation of 1094 deaths in Sweden during 1995. J Clin Epidemiol 62(11):1202–1209
Eccles SA, Aboagye EO, Ali S, Anderson AS, Armes J, Berditchevski F, Blaydes JP, Brennan K, Brown NJ, Bryant HE, Bundred NJ, Burchell JM, Campbell AM, Carroll JS, Clarke RB, Coles CE, Cook GJ, Cox A, Curtin NJ, Dekker LV, Silva Idos S, Duffy SW, Easton DF, Eccles DM, Edwards DR, Edwards J, Evans D, Fenlon DF, Flanagan JM, Foster C, Gallagher WM, Garcia-Closas M, Gee JM, Gescher AJ, Goh V, Groves AM, Harvey AJ, Harvie M, Hennessy BT, Hiscox S, Holen I, Howell SJ, Howell A, Hubbard G, Hulbert-Williams N, Hunter MS, Jasani B, Jones LJ, Key TJ, Kirwan CC, Kong A, Kunkler IH, Langdon SP, Leach MO, Mann DJ, Marshall JF, Martin L, Martin SG, Macdougall JE, Miles DW, Miller WR, Morris JR, Moss SM, Mullan P, Natrajan R, O’Connor JP, O’Connor R, Palmieri C, Pharoah PD, Rakha EA, Reed E, Robinson SP, Sahai E, Saxton JM, Schmid P, Smalley MJ, Speirs V, Stein R, Stingl J, Streuli CH, Tutt AN, Velikova G, Walker RA, Watson CJ, Williams KJ, Young LS, Thompson AM (2013) Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res 15(5):R92
Sestak I, Dowsett M, Zabaglo L, Lopez-Knowles E, Ferree S, Cowens JW, Cuzick J (2013) Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst 105(19):1504–1511
Bonnefoi H, Grellety T, Tredan O, Saghatchian M, Dalenc F, Mailliez A, L’Haridon T, Cottu P, Abadie-Lacourtoisie S, You B, Mousseau M, Dauba J, Del Piano F, Desmoulins I, Coussy F, Madranges N, Grenier J, Bidard FC, Proudhon C, MacGrogan G, Orsini C, Pulido M, Goncalves A (2016) A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1). Ann Oncol 27(5):812–818
Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, Blackwell K, Rugo H, Nabell L, Forero A, Stearns V, Doane AS, Danso M, Moynahan ME, Momen LF, Gonzalez JM, Akhtar A, Giri DD, Patil S, Feigin KN, Hudis CA, Traina TA, Translational Breast Cancer Research C (2013) Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res 19(19):5505–5512
Traina TA, Yardley DA, Patel MR, Schwartzberg LS, Elias A, Gucalp A, Blaney ME, Gibbons J, Hudis CA, LoRusso P (2014) 10p A phase 1 open-label study evaluating the safety, tolerability, and pharmacokinetics of enzalutamide alone or combined with an aromatase inhibitor in women with advanced breast cancer. Ann Oncol 25(suppl 1):i4
Traina T, O’Shaughnessy J, Kelly C, Schwartzberg L, Gucalp A, Peterson A, Bhattacharya S, Trudeau M, Hudis C, Schmid P (2013) Abstract OT3-2-08: A phase 2 single-arm study of the clinical activity and safety of enzalutamide in patients with advanced androgen receptor-positive triple-negative breast cancer. Cancer Res 73(24 Supplement):OT3-2-08
De Amicis F, Thirugnansampanthan J, Cui Y, Selever J, Beyer A, Parra I, Weigel NL, Herynk MH, Tsimelzon A, Lewis MT, Chamness GC, Hilsenbeck SG, Ando S, Fuqua SA (2010) Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat 121(1):1–11
Rechoum Y, Rovito D, Iacopetta D, Barone I, Andò S, Weigel N, O’Malley B, Brown P, Fuqua SW (2014) AR collaborates with ERα in aromatase inhibitor-resistant breast cancer. Breast Cancer Res Treat 147(3):473–485
Ciupek A, Rechoum Y, Gu G, Gelsomino L, Beyer AR, Brusco L, Covington KR, Tsimelzon A, Fuqua SA (2015) Androgen receptor promotes tamoxifen agonist activity by activation of EGFR in ERalpha-positive breast cancer. Breast Cancer Res Treat 154(2):225–237
Barton VN, D’Amato NC, Gordon MA, Lind HT, Spoelstra NS, Babbs BL, Heinz RE, Elias A, Jedlicka P, Jacobsen BM, Richer JK (2015) Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther 14(3):769–778
D’Amato NC, Gordon MA, Babbs B, Spoelstra NS, Carson Butterfield KT, Torkko KC, Phan VT, Barton VN, Rogers TJ, Sartorius CA, Elias A, Gertz J, Jacobsen BM, Richer JK (2016) Cooperative dynamics of AR and ER activity in breast cancer. Mol Cancer Res 14(11):1054–1067
Gucalp A, Traina TA (2017) Androgen receptor-positive, triple-negative breast cancer. Cancer 123(10):1686–1688
Gordon MA, D’Amato NC, Gu H, Babbs B, Wulfkuhle JD, Petricoin EF, Gallagher I, Dong T, Torkko K, Liu B, Elias A, Richer JK (2017) Synergy between androgen receptor antagonism and inhibition of mTOR and HER2 in breast cancer. Mol Cancer Therapeutics. doi:10.1158/1535-7163.MCT-17-0111
Acknowledgements
The authors thank Elise Nilsson (TMA construction), Kristina Lövgren (sectioning, staining, histopathologic assessments), breast pathologists Anna Ehinger and Lola Anagnostaki (histopathologic assessments), research colleagues in the MDCS Breast Cancer network (data collection), and MDCS data manager Anna Hwasser.
Funding
The study was funded by Governmental Funding for Clinical Research within the National Health Services (Region Skåne ALF), The Swedish Cancer Society, and the Swedish Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
S Borgquist has received consultant honario from Roche and Novartis. The other authors declare that they have no competing interests.
Ethical standards
Ethical permission was obtained from the Ethical Committee at Lund University (Dnr 472/2007). All participants signed a written informed consent form.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Elebro, K., Bendahl, PO., Jernström, H. et al. Androgen receptor expression and breast cancer mortality in a population-based prospective cohort. Breast Cancer Res Treat 165, 645–657 (2017). https://doi.org/10.1007/s10549-017-4343-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4343-0