Abstract
Purpose
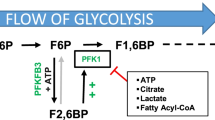
Human epidermal growth factor receptor-2 (HER2) has been implicated in the progression of multiple tumor types, including breast cancer, and many downstream effectors of HER2 signaling are primary regulators of cellular metabolism, including Ras and Akt. A key downstream metabolic target of Ras and Akt is the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 isozyme (PFKFB3), whose product, fructose-2,6-bisphosphate (F26BP), is a potent allosteric activator of a rate-limiting enzyme in glycolysis, 6-phosphofructo-1-kinase (PFK-1). We postulate that PFKFB3 may be regulated by HER2 and contribute to HER2-driven tumorigenicity.
Methods
Immunohistochemistry and Kaplan–Meier analysis of HER2+ patient samples investigated the relevance of PFKFB3 in HER2+ breast cancer. In vitro genetic and pharmacological inhibition of PFKFB3 was utilized to determine effects on HER2+ breast cancer cells, while HER2 antagonist treatment assessed the mechanistic regulation on PFKFB3 expression and glucose metabolism. Administration of a PFKFB3 inhibitor in a HER2-driven transgenic breast cancer model evaluated this potential therapeutic approach in vivo.
Results
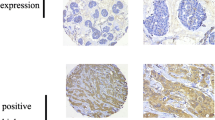
PFKFB3 is elevated in human HER2+ breast cancer and high PFKFB3 transcript correlated with poorer progression-free (PFS) and distant metastatic-free (DFMS) survival. Constitutive HER2 expression led to elevated PFKFB3 expression and increased glucose metabolism, while inhibition of PFKFB3 suppressed glucose uptake, F26BP, glycolysis, and selectively decreased the growth of HER2-expressing breast cancer cells. In addition, treatment with lapatinib, an FDA-approved HER2 inhibitor, decreased PFKFB3 expression and glucose metabolism in HER2+ cells. In vivo administration of a PFKFB3 antagonist significantly suppressed the growth of HER2-driven breast tumors and decreased 18F-2-deoxy-glucose uptake.
Conclusions
Taken together, these data support the potential clinical utility of PFKFB3 inhibitors as chemotherapeutic agents against HER2+ breast cancer.









Similar content being viewed by others
References
Castell F, Cook GJ (2008) Quantitative techniques in 18FDG PET scanning in oncology. Br J Cancer 98(10):1597–1601
Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11(2):85–95
Dang CV (2012) Links between metabolism and cancer. Genes Dev 26(9):877–890
Jones RG, Thompson CB (2009) Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev 23(5):537–548
Levine AJ, Puzio-Kuter AM (2010) The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330(6009):1340–1344
McCann AH, Dervan PA, O’Regan M, Codd MB, Gullick WJ, Tobin BM, Carney DN (1991) Prognostic significance of c-erbB-2 and estrogen receptor status in human breast cancer. Cancer Res 51(12):3296–3303
van de Vijver M, van de Bersselaar R, Devilee P, Cornelisse C, Peterse J, Nusse R (1987) Amplification of the neu (c-erbB-2) oncogene in human mammmary tumors is relatively frequent and is often accompanied by amplification of the linked c-erbA oncogene. Mol Cell Biol 7(5):2019–2023
Hynes NE, Stern DF (1994) The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta 1198(2–3):165–184
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Chesney J, Telang S (2013) Regulation of glycolytic and mitochondrial metabolism by ras. Curr Pharm Biotechnol 14(3):251–260
Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM et al (2004) Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64(11):3892–3899
Robey RB, Hay N (2009) Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol 19(1):25–31
Zhao YH, Zhou M, Liu H, Ding Y, Khong HT, Yu D, Fodstad O, Tan M (2009) Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene 28(42):3689–3701
Cheyne RW, Trembleau L, McLaughlin A, Smith TA (2011) Changes in 2-fluoro-2-deoxy-d-glucose incorporation, hexokinase activity and lactate production by breast cancer cells responding to treatment with the anti-HER-2 antibody trastuzumab. Nucl Med Biol 38(3):339–346
Van Schaftingen E, Hue L, Hers HG (1980) Control of the fructose-6-phosphate/fructose 1,6-bisphosphate cycle in isolated hepatocytes by glucose and glucagon. Role of a low-molecular-weight stimulator of phosphofructokinase. Biochem J 192(3):887–895
Van Schaftingen E, Hue L, Hers HG (1980) Fructose 2,6-bisphosphate, the probably structure of the glucose- and glucagon-sensitive stimulator of phosphofructokinase. Biochem J 192(3):897–901
Yalcin A, Telang S, Clem B, Chesney J (2009) Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol 86(3):174–179
Ros S, Schulze A (2013) Balancing glycolytic flux: the role of 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatases in cancer metabolism. Cancer & metabolism 1(1):8
Sakakibara R, Kato M, Okamura N, Nakagawa T, Komada Y, Tominaga N, Shimojo M, Fukasawa M (1997) Characterization of a human placental fructose-6-phosphate, 2-kinase/fructose-2,6-bisphosphatase. J Biochem 122(1):122–128
Atsumi T, Chesney J, Metz C, Leng L, Donnelly S, Makita Z, Mitchell R, Bucala R (2002) High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res 62(20):5881–5887
Telang S, Yalcin A, Clem AL, Bucala R, Lane AN, Eaton JW, Chesney J (2006) Ras transformation requires metabolic control by 6-phosphofructo-2-kinase. Oncogene 25(55):7225–7234
Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, Rasku MA, Arumugam S, Dean WL, Eaton J et al (2008) Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther 7(1):110–120
Clem BF, O’Neal J, Tapolsky G, Clem AL, Imbert-Fernandez Y, Kerr DA 2nd, Klarer AC, Redman R, Miller DM, Trent JO et al (2013) Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther 12(8):1461–1470
Van Schaftingen E, Lederer B, Bartrons R, Hers HG (1982) A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem 129(1):191–195
Imbert-Fernandez Y, Clem BF, O’Neal J, Kerr DA, Spaulding R, Lanceta L, Clem AL, Telang S, Chesney J (2014) Estradiol stimulates glucose metabolism via 6-phosphofructo-2-kinase (PFKFB3). J Biol Chem 289(13):9440–9448
Taetle R, Rosen F, Abramson I, Venditti J, Howell S (1987) Use of nude mouse xenografts as preclinical drug screens: in vivo activity of established chemotherapeutic agents against melanoma and ovarian carcinoma xenografts. Cancer Treat Rep 71(3):297–304
Chesney J, Clark J, Klarer AC, Imbert-Fernandez Y, Lane AN, Telang S (2014) Fructose-2,6-bisphosphate synthesis by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 (PFKFB4) is required for the glycolytic response to hypoxia and tumor growth. Oncotarget 5(16):6670–6686
Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z (2010) An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1809 patients. Breast Cancer Res Treat 123(3):725–731
Hudis CA (2007) Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med 357(1):39–51
Cantor JR, Sabatini DM (2012) Cancer cell metabolism: one hallmark, many faces. Cancer discovery 2(10):881–898
Gravalos C, Jimeno A (2008) HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann oncol 19(9):1523–1529
Shojaei S, Gardaneh M, Shamabadi AR (2012) Target points in trastuzumab resistance. Intl J breast cancer 2012:761917
Almeida A, Bolanos JP, Moncada S (2010) E3 ubiquitin ligase APC/C-Cdh1 accounts for the Warburg effect by linking glycolysis to cell proliferation. Proc Natl Acad Sci U S A 107(2):738–741
Bando H, Atsumi T, Nishio T, Niwa H, Mishima S, Shimizu C, Yoshioka N, Bucala R, Koike T (2005) Phosphorylation of the 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase/PFKFB3 family of glycolytic regulators in human cancer. Clin Cancer Res 11(16):5784–5792
Zhao Y, Liu H, Liu Z, Ding Y, Ledoux SP, Wilson GL, Voellmy R, Lin Y, Lin W, Nahta R et al (2011) Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res 71(13):4585–4597
Clem BF, O’Neal J, Klarer AC, Telang S, Chesney J (2015) Clinical development of cancer therapeutics that target metabolism. QJM 109(6):367–372
Thornburg JM, Nelson KK, Clem BF, Lane AN, Arumugam S, Simmons A, Eaton JW, Telang S, Chesney J (2008) Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res 10(5):R84
Acknowledgments
This work was supported by the Department of Defense CDMRP (BC112204 to JO), the National Cancer Institute [CA166327 to BFC; CA149438 to JC], and by the American Cancer Society (RSG 13-139-01-CNE to BFC). We gratefully acknowledge Drs. Chin Ng and Huaiyu Zheng within the James Graham Brown Cancer Center (JGBCC) animal imaging core facility for assistance with the microCT and microPET procedures and Dr. Senthil Muthuswamy for providing the MCF10A-HER2 cells.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ST, JC, and BFC are co-inventors on patents related to PFKFB3 inhibitors (US Patent#8,088,385) which are wholly owned by the University of Louisville Research Foundation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
O’Neal, J., Clem, A., Reynolds, L. et al. Inhibition of 6-phosphofructo-2-kinase (PFKFB3) suppresses glucose metabolism and the growth of HER2+ breast cancer. Breast Cancer Res Treat 160, 29–40 (2016). https://doi.org/10.1007/s10549-016-3968-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3968-8