Abstract
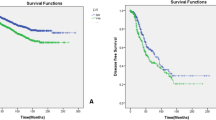
Various prognostic indicators have been investigated in neoadjuvant chemotherapy (NAC)-treated invasive breast cancer (BC). Our study examines if lymphovascular invasion (LVI) is an independent predictor of survival in women receiving NAC. We performed a retrospective analysis in 166 women with operable invasive BC who underwent adriamycin- and taxane-based NAC between 2000 and 2013. The presence of LVI was noted in breast excisions following NAC. Associations between progression-free and overall survival and LVI and other clinicopathologic variables were assessed. Median follow-up was 31 months (range 1.4–153 months) with a total of 56 events and 24 deaths from any cause. LVI was found in 74 of 166 patients (45 %). In univariate analysis, the presence of LVI was associated with worse progression-free survival (HR 3.37, 95 % CI 1.87–6.06, p < 0.01) and overall survival (HR 4.35, 95 % CI 1.61–11.79, p < 0.01). In multivariate models adjusting for breast cancer subtype, LVI was significantly associated with a decrease in progression-free survival (HR 3.76, 95 % CI 2.07–6.83, p < 0.01) and overall survival (HR 5.70, 95 % CI 2.08–15.64, p < 0.01). When stratified by subtype, those with hormone receptor or HER2-positive BCs with no LVI had the most favorable progression-free and overall survival. Those with both LVI and triple-negative BC had the worst progression-free and overall survival. LVI is an important prognostic marker and is associated with worse clinical outcome in breast cancer patients receiving NAC.



Similar content being viewed by others
Abbreviations
- LVI:
-
Lymphovascular invasion
- NAC:
-
Neoadjuvant chemotherapy
- TNBC:
-
Triple-negative breast cancer
- pCR:
-
Pathological complete response
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
References
Ragaz J (1986) Preoperative (neoadjuvant) chemotherapy for breast cancer: outline of the British Columbia Trial. Recent Results Cancer Res 103:85–94
Ragaz J, Baird R, Rebbeck P, Goldie A, Coldman A, Spinelli J (1985) Preoperative adjuvant chemotherapy (neoadjuvant) for carcinoma of the breast: rationale and safety report. Recent Results Cancer Res 98:99–105
Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, Buzdar AU, Smith IE, Symmans WF, Singh B, Winer EP (2008) Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol 26(5):814–819. doi:10.1200/JCO.2007.15.3510
Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, Blohmer JU, Eiermann W, Jackesz R, Jonat W, Lebeau A, Loibl S, Miller W, Seeber S, Semiglazov V, Smith R, Souchon R, Stearns V, Untch M, von Minckwitz G (2006) Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol 24(12):1940–1949. doi:10.1200/JCO.2005.02.6187
Tamura N, Hasebe T, Okada N, Houjoh T, Akashi-Tanaka S, Shimizu C, Shibata T, Sasajima Y, Iwasaki M, Kinoshita T (2009) Tumor histology in lymph vessels and lymph nodes for the accurate prediction of outcome among breast cancer patients treated with neoadjuvant chemotherapy. Cancer Sci 100(10):1823–1833. doi:10.1111/j.1349-7006.2009.01264.x
Petit T, Borel C, Ghnassia JP, Rodier JF, Escande A, Mors R, Haegele P (2001) Chemotherapy response of breast cancer depends on HER-2 status and anthracycline dose intensity in the neoadjuvant setting. Clin Cancer Res 7(6):1577–1581
Keskin S, Muslumanoglu M, Saip P, Karanlik H, Guveli M, Pehlivan E, Aydogan F, Eralp Y, Aydiner A, Yavuz E, Ozmen V, Igci A, Topuz E (2011) Clinical and pathological features of breast cancer associated with the pathological complete response to anthracycline-based neoadjuvant chemotherapy. Oncology 81(1):30–38. doi:10.1159/000330766
Chollet P, Amat S, Cure H, de Latour M, Le Bouedec G, Mouret-Reynier MA, Ferriere JP, Achard JL, Dauplat J, Penault-Llorca F (2002) Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer 86(7):1041–1046. doi:10.1038/sj.bjc.6600210
Penault-Llorca F, Vincent-Salomon A (2003) Roles of the pathologist in neoadjuvant chemotherapy: evaluation of response, prognostic and predictive factors. Ann Pathol 23(6):555–563
Bollet MA, Sigal-Zafrani B, Gambotti L, Extra JM, Meunier M, Nos C, Dendale R, Campana F, Kirova YM, Dieras V, Fourquet A, Institut Curie Breast Cancer Study G (2006) Pathological response to preoperative concurrent chemo-radiotherapy for breast cancer: results of a phase II study. Eur J Cancer 42(14):2286–2295. doi:10.1016/j.ejca.2006.03.026
Ferriere JP, Assier I, Cure H, Charrier S, Kwiatkowski F, Achard JL, Dauplat J, Chollet P (1998) Primary chemotherapy in breast cancer: correlation between tumor response and patient outcome. Am J Clin Oncol 21(2):117–120
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, Buzdar AU, Singletary SE, Hortobagyi GN, Pusztai L (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25(28):4414–4422. doi:10.1200/JCO.2007.10.6823
Rody A, Karn T, Gatje R, Ahr A, Solbach C, Kourtis K, Munnes M, Loibl S, Kissler S, Ruckhaberle E, Holtrich U, von Minckwitz G, Kaufmann M (2007) Gene expression profiling of breast cancer patients treated with docetaxel, doxorubicin, and cyclophosphamide within the GEPARTRIO trial: HER-2, but not topoisomerase II alpha and microtubule-associated protein tau, is highly predictive of tumor response. Breast 16(1):86–93. doi:10.1016/j.breast.2006.06.008
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172. doi:10.1016/s0140-6736(13)62422-8
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30(15):1796–1804. doi:10.1200/JCO.2011.38.8595
Mohammed RA, Martin SG, Gill MS, Green AR, Paish EC, Ellis IO (2007) Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am J Surg Pathol 31(12):1825–1833. doi:10.1097/PAS.0b013e31806841f6
Song YJ, Shin SH, Cho JS, Park MH, Yoon JH, Jegal YJ (2011) The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. J breast cancer 14(3):198–203. doi:10.4048/jbc.2011.14.3.198
Karaman S, Detmar M (2014) Mechanisms of lymphatic metastasis. J Clin Invest 124(3):922–928. doi:10.1172/JCI71606
Lee AH, Pinder SE, Macmillan RD, Mitchell M, Ellis IO, Elston CW, Blamey RW (2006) Prognostic value of lymphovascular invasion in women with lymph node negative invasive breast carcinoma. Eur J Cancer 42(3):357–362. doi:10.1016/j.ejca.2005.10.021
Mohammed RA, Martin SG, Mahmmod AM, Macmillan RD, Green AR, Paish EC, Ellis IO (2011) Objective assessment of lymphatic and blood vascular invasion in lymph node-negative breast carcinoma: findings from a large case series with long-term follow-up. J Pathol 223(3):358–365. doi:10.1002/path.2810
Pinder SE, Ellis IO, Galea M, O’Rouke S, Blamey RW, Elston CW (1994) Pathological prognostic factors in breast cancer. III. Vascular invasion: relationship with recurrence and survival in a large study with long-term follow-up. Histopathology 24(1):41–47
Rakha EA, Martin S, Lee AH, Morgan D, Pharoah PD, Hodi Z, Macmillan D, Ellis IO (2012) The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer 118(15):3670–3680. doi:10.1002/cncr.26711
Freedman GM, Li T, Polli LV, Anderson PR, Bleicher RJ, Sigurdson E, Swaby R, Dushkin H, Patchefsky A, Goldstein L (2012) Lymphatic space invasion is not an independent predictor of outcomes in early stage breast cancer treated by breast-conserving surgery and radiation. Breast J 18(5):415–419. doi:10.1111/j.1524-4741.2012.01271.x
Ejlertsen B, Jensen MB, Rank F, Rasmussen BB, Christiansen P, Kroman N, Kvistgaard ME, Overgaard M, Toftdahl DB, Mouridsen HT (2009) Population-based study of peritumoral lymphovascular invasion and outcome among patients with operable breast cancer. J Natl Cancer Inst 101(10):729–735. doi:10.1093/jnci/djp090
Uematsu T, Kasami M, Watanabe J, Takahashi K, Yamasaki S, Tanaka K, Tadokoro Y, Ogiya A (2011) Is lymphovascular invasion degree one of the important factors to predict neoadjuvant chemotherapy efficacy in breast cancer? Breast cancer 18(4):309–313. doi:10.1007/s12282-010-0211-z
Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S (2010) American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J oncol pract 6(4):195–197. doi:10.1200/jop.777003
Tchrakian N, Flanagan L, Harford J, Gannon JM, Quinn CM (2015) New ASCO/CAP guideline recommendations for HER2 testing increase the proportion of reflex in situ hybridization tests and of HER2 positive breast cancers. Virchows Archiv. doi:10.1007/s00428-015-1871-z
Zhang C, Wang S, Israel HP, Yan SX, Horowitz DP, Crockford S, Gidea-Addeo D, Clifford Chao KS, Kalinsky K, Connolly EP (2015) Higher locoregional recurrence rate for triple-negative breast cancer following neoadjuvant chemotherapy, surgery and radiotherapy. SpringerPlus 4:386. doi:10.1186/s40064-015-1116-2
Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE Jr, Taghian A, Wickerham DL, Wolmark N (2012) Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of national surgical adjuvant breast and bowel project B-18 and B-27. J Clin Oncol 30(32):3960–3966. doi:10.1200/JCO.2011.40.8369
Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman J-AW, Sparano JA, Hunsberger S, Enos RA, Gelber RD (2007) Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 25(15):2127–2132
Abdel-Fatah TM, Ball G, Lee AH, Pinder S, MacMilan RD, Cornford E, Moseley PM, Silverman R, Price J, Latham B, Palmer D, Chan A, Ellis IO, Chan SY (2015) Nottingham Clinico-Pathological Response Index (NPRI) after neoadjuvant chemotherapy (Neo-ACT) accurately predicts clinical outcome in locally advanced breast cancer. Clinical Cancer Res 21(5):1052–1062. doi:10.1158/1078-0432.ccr-14-0685
Caudle AS, Yu TK, Tucker SL, Bedrosian I, Litton JK, Gonzalez-Angulo AM, Hoffman K, Meric-Bernstam F, Hunt KK, Buchholz TA, Mittendorf EA (2012) Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res 14(3):R83. doi:10.1186/bcr3198
Huang EH, Tucker SL, Strom EA, McNeese MD, Kuerer HM, Hortobagyi GN, Buzdar AU, Valero V, Perkins GH, Schechter NR, Hunt KK, Sahin AA, Buchholz TA (2005) Predictors of locoregional recurrence in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy, mastectomy, and radiotherapy. Int J Radiat Oncol Biol Phys 62(2):351–357. doi:10.1016/j.ijrobp.2004.09.056
Sakuma K, Kurosumi M, Oba H, Kobayashi Y, Takei H, Inoue K, Tabei T, Oyama T (2011) Pathological tumor response to neoadjuvant chemotherapy using anthracycline and taxanes in patients with triple-negative breast cancer. Exp Ther Med 2(2):257–264. doi:10.3892/etm.2011.212
Schoppmann SF, Bayer G, Aumayr K, Taucher S, Geleff S, Rudas M, Kubista E, Hausmaninger H, Samonigg H, Gnant M, Jakesz R, Horvat R, Austrian B, Colorectal Cancer Study G (2004) Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg 240(2):306–312
Kerjaschki D, Bago-Horvath Z, Rudas M, Sexl V, Schneckenleithner C, Wolbank S, Bartel G, Krieger S, Kalt R, Hantusch B, Keller T, Nagy-Bojarszky K, Huttary N, Raab I, Lackner K, Krautgasser K, Schachner H, Kaserer K, Rezar S, Madlener S, Vonach C, Davidovits A, Nosaka H, Hammerle M, Viola K, Dolznig H, Schreiber M, Nader A, Mikulits W, Gnant M, Hirakawa S, Detmar M, Alitalo K, Nijman S, Offner F, Maier TJ, Steinhilber D, Krupitza G (2011) Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J Clin Invest 121(5):2000–2012. doi:10.1172/JCI44751
Zhang ZQ, Han YZ, Nian Q, Chen G, Cui SQ, Wang XY (2015) Tumor Invasiveness, Not Lymphangiogenesis, Is Correlated with Lymph Node Metastasis and Unfavorable Prognosis in Young Breast Cancer Patients (</=35 Years). PLoS One 10(12):e0144376. doi:10.1371/journal.pone.0144376
Mohammed RA, Ellis IO, Mahmmod AM, Hawkes EC, Green AR, Rakha EA, Martin SG (2011) Lymphatic and blood vessels in basal and triple-negative breast cancers: characteristics and prognostic significance. Mod Pathol 24(6):774–785. doi:10.1038/modpathol.2011.4
Niemiec J, Adamczyk A, Ambicka A, Mucha-Malecka A, Wysocki W, Mitus J, Rys J (2012) Lymphangiogenesis assessed using three methods is related to tumour grade, breast cancer subtype and expression of basal marker. Pol J Pathol 63(3):165–171
Acknowledgments
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number KL2 TR000081. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the above authors have any conflicts of interest to declare.
Additional information
Ying L. Liu and Anurag Saraf—co authors.
Rights and permissions
About this article
Cite this article
Liu, Y.L., Saraf, A., Lee, S.M. et al. Lymphovascular invasion is an independent predictor of survival in breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat 157, 555–564 (2016). https://doi.org/10.1007/s10549-016-3837-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3837-5