Abstract
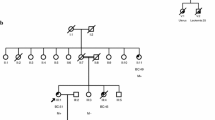
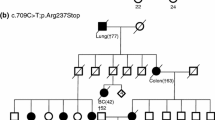
Germ-line mutations in the RAD51C gene have recently been identified in families with breast and ovarian cancer and have been associated with an increased risk of ovarian cancer. In this study, we describe the frequency of pathogenic RAD51C mutations identified in Danish breast and/or ovarian cancer families. We screened the RAD51C gene in 1228 Danish hereditary breast and/or ovarian cancer families by next-generation sequencing analysis. The frequency of the identified variants was examined in the exome sequencing project database and in data from 2000 Danish exomes and the presumed significance of missense and intronic variants was predicted by in silico analysis. We identified six families with a pathogenic mutation in RAD51C, including three frameshift mutations, one nonsense mutation, and 2 missense mutations. Overall, pathogenic RAD51C mutations were identified in 0.5 % of Danish families with increased risk of hereditary breast and/or ovarian cancer. Moreover, we identified 24 additional RAD51C variants of which 14 have not been previously reported in the literature. In this study, we determine the prevalence of RAD51C mutations in Danish breast and/or ovarian cancer families. We identified six pathogenic RAD51C mutations as well as 23 variants of uncertain clinical significance and one benign variant. Together, the study extends our knowledge of the RAD51C mutation spectrum and supports that RAD51C should be included in gene panel testing of individuals with high risk of breast and ovarian cancer.

Similar content being viewed by others
References
Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A et al (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72(5):1117–1130
King MC, Marks JH, Mandell JB (2003) Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 302(5645):643–646
van der Kolk DM, de Bock GH, Leegte BK, Schaapveld M, Mourits MJ, de Vries J, van der Hout AH, Oosterwijk JC (2010) Penetrance of breast cancer, ovarian cancer and contralateral breast cancer in BRCA1 and BRCA2 families: high cancer incidence at older age. Breast Cancer Res Treat 124(3):643–651
Hansen T, Jonson L, Albrechtsen A, Andersen MK, Ejlertsen B, Nielsen FC (2009) Large BRCA1 and BRCA2 genomic rearrangements in Danish high risk breast-ovarian cancer families. Breast Cancer Res Treat 115(2):315–323
Hansen TV, Bisgaard ML, Jonson L, Albrechtsen A, Filtenborg-Barnkob B, Eiberg H, Ejlertsen B, Nielsen FC (2008) Novel de novo BRCA2 mutation in a patient with a family history of breast cancer. BMC Med Genet 9:58
Hansen TV, Ejlertsen B, Albrechtsen A, Bergsten E, Bjerregaard P, Hansen T, Myrhoj T, Nielsen PB, Timmermans-Wielenga V, Andersen MK et al (2009) A common Greenlandic Inuit BRCA1 RING domain founder mutation. Breast Cancer Res Treat 115(1):69–76
Hansen TV, Jonson L, Albrechtsen A, Steffensen AY, Bergsten E, Myrhoj T, Ejlertsen B, Nielsen FC (2010) Identification of a novel BRCA1 nucleotide 4803delCC/c.4684delCC mutation and a nucleotide 249T > A/c.130T > A (p.Cys44Ser) mutation in two Greenlandic Inuit families: implications for genetic screening of Greenlandic Inuit families with high risk for breast and/or ovarian cancer. Breast Cancer Res Treat 124(1):259–264
Hansen TV, Jonson L, Steffensen AY, Andersen MK, Kjaergaard S, Gerdes AM, Ejlertsen B, Nielsen FC (2011) Screening of 1331 Danish breast and/or ovarian cancer families identified 40 novel BRCA1 and BRCA2 mutations. Fam Cancer 10(2):207–212
Steffensen AY, Dandanell M, Jonson L, Ejlertsen B, Gerdes AM, Nielsen FC, Hansen T (2014) Functional characterization of BRCA1 gene variants by mini-gene splicing assay. Eur J Hum Genet 22(12):1362–1368
Steffensen AY, Jonson L, Ejlertsen B, Gerdes AM, Nielsen FC, Hansen TV (2010) Identification of a Danish breast/ovarian cancer family double heterozygote for BRCA1 and BRCA2 mutations. Fam Cancer 9(3):283–287
Thomassen M, Blanco A, Montagna M, Hansen TV, Pedersen IS, Gutierrez-Enriquez S, Menendez M, Fachal L, Santamarina M, Steffensen AY et al (2012) Characterization of BRCA1 and BRCA2 splicing variants: a collaborative report by ENIGMA consortium members. Breast Cancer Res Treat 132(3):1009–1023
Thomassen M, Hansen TV, Borg A, Lianee HT, Wikman F, Pedersen IS, Bisgaard ML, Nielsen FC, Kruse TA, Gerdes AM (2008) BRCA1 and BRCA2 mutations in Danish families with hereditary breast and/or ovarian cancer. Acta Oncol 47(4):772–777
Thomassen M, Pedersen IS, Vogel I, Hansen TV, Brasch-Andersen C, Brasen CL, Cruger D, Sunde L, Nielsen FC, Jensen UB et al (2011) A BRCA2 mutation incorrectly mapped in the original BRCA2 reference sequence, is a common West Danish founder mutation disrupting mRNA splicing. Breast Cancer Res Treat 128(1):179–185
Guilford PJ, Hopkins JB, Grady WM, Markowitz SD, Willis J, Lynch H, Rajput A, Wiesner GL, Lindor NM, Burgart LJ et al (1999) E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat 14(3):249–255
Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M et al (1997) Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet 16(1):64–67
Meindl A, Hellebrand H, Wiek C, Erven V, Wappenschmidt B, Niederacher D, Freund M, Lichtner P, Hartmann L, Schaal H et al (2010) Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet 42(5):410–414
Sidransky D, Tokino T, Helzlsouer K, Zehnbauer B, Rausch G, Shelton B, Prestigiacomo L, Vogelstein B, Davidson N (1992) Inherited p53 gene mutations in breast cancer. Cancer Res 52(10):2984–2986
Suwaki N, Klare K, Tarsounas M (2011) RAD51 paralogs: roles in DNA damage signalling, recombinational repair and tumorigenesis. Semin Cell Dev Biol 22(8):898–905
Ahlborn LB, Steffensen AY, Jonson L, Djursby M, Nielsen FC, Gerdes AM, Hansen TV (2015) Identification of a breast cancer family double heterozygote for RAD51C and BRCA2 gene mutations. Fam Cancer 14(1):129–133
Akbari MR, Tonin P, Foulkes WD, Ghadirian P, Tischkowitz M, Narod SA (2010) RAD51C germline mutations in breast and ovarian cancer patients. Breast Cancer Res 12(4):404
Blanco A, Gutierrez-Enriquez S, Santamarina M, Montalban G, Bonache S, Balmana J, Carracedo A, Diez O, Vega A (2014) RAD51C germline mutations found in Spanish site-specific breast cancer and breast-ovarian cancer families. Breast Cancer Res Treat 147(1):133–143
Clague J, Wilhoite G, Adamson A, Bailis A, Weitzel JN, Neuhausen SL (2011) RAD51C germline mutations in breast and ovarian cancer cases from high-risk families. Plos One 6(9):e25632
Coulet F, Fajac A, Colas C, Eyries M, Dion-Miniere A, Rouzier R, Uzan S, Lefranc JP, Carbonnel M, Cornelis F et al (2013) Germline RAD51C mutations in ovarian cancer susceptibility. Clin Genet 83(4):332–336
Cunningham JM, Cicek MS, Larson NB, Davila J, Wang C, Larson MC, Song H, Dicks EM, Harrington P, Wick M et al (2014) Clinical characteristics of ovarian cancer classified by BRCA1, BRCA2, and RAD51C status. Sci Rep 4:4026
De Leeneer K, Van Bockstal M, De Brouwer S, Swietek N, Schietecatte P, Sabbaghian N, Van den Ende J, Willocx S, Storm K, Blaumeiser B et al (2012) Evaluation of RAD51C as cancer susceptibility gene in a large breast-ovarian cancer patient population referred for genetic testing. Breast Cancer Res Treat 133(1):393–398
Kushnir A, Laitman Y, Shimon SP, Berger R, Friedman E (2012) Germline mutations in RAD51C in Jewish high cancer risk families. Breast Cancer Res Treat 136(3):869–874
Loveday C, Turnbull C, Ruark E, Xicola RM, Ramsay E, Hughes D, Warren-Perry M, Snape K, Eccles D, Evans DG et al (2012) Germline RAD51C mutations confer susceptibility to ovarian cancer. Nat Genet 44(5):475–476; author reply 476
Lu W, Wang X, Lin H, Lindor NM, Couch FJ (2012) Mutation screening of RAD51C in high-risk breast and ovarian cancer families. Fam Cancer 11(3):381–385
Osorio A, Endt D, Fernandez F, Eirich K, de la Hoya M, Schmutzler R, Caldes T, Meindl A, Schindler D, Benitez J (2012) Predominance of pathogenic missense variants in the RAD51C gene occurring in breast and ovarian cancer families. Hum Mol Genet 21(13):2889–2898
Pang Z, Yao L, Zhang J, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, Xie Y (2011) RAD51C germline mutations in Chinese women with familial breast cancer. Breast Cancer Res Treat 129(3):1019–1020
Pelttari LM, Heikkinen T, Thompson D, Kallioniemi A, Schleutker J, Holli K, Blomqvist C, Aittomaki K, Butzow R, Nevanlinna H (2011) RAD51C is a susceptibility gene for ovarian cancer. Hum Mol Genet 20(16):3278–3288
Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, Thornton A, Norquist BM, Casadei S, Nord AS et al (2014) Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res 20(3):764–775
Rashid MU, Muhammad N, Faisal S, Amin A, Hamann U (2014) Deleterious RAD51C germline mutations rarely predispose to breast and ovarian cancer in Pakistan. Breast Cancer Res Treat 145(3):775–784
Romero A, Perez-Segura P, Tosar A, Garcia-Saenz JA, Diaz-Rubio E, Caldes T, de la Hoya M (2011) A HRM-based screening method detects RAD51C germ-line deleterious mutations in Spanish breast and ovarian cancer families. Breast Cancer Res Treat 129(3):939–946
Schnurbein G, Hauke J, Wappenschmidt B, Weber-Lassalle N, Engert S, Hellebrand H, Garbes L, Becker A, Neidhardt G, Rhiem K et al (2013) RAD51C deletion screening identifies a recurrent gross deletion in breast cancer and ovarian cancer families. Breast Cancer Res 15(6):R120
Silvestri V, Rizzolo P, Falchetti M, Zanna I, Masala G, Palli D, Ottini L (2011) Mutation screening of RAD51C in male breast cancer patients. Breast Cancer Res 13(1):404
Thompson ER, Boyle SE, Johnson J, Ryland GL, Sawyer S, Choong DY, kConFab, Chenevix-Trench G, Trainer AH, Lindeman GJ et al (2012) Analysis of RAD51C germline mutations in high-risk breast and ovarian cancer families and ovarian cancer patients. Hum Mutat 33(1):95–99
Vaz F, Hanenberg H, Schuster B, Barker K, Wiek C, Erven V, Neveling K, Endt D, Kesterton I, Autore F et al (2010) Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nat Genet 42(5):406–409
Vuorela M, Pylkas K, Hartikainen JM, Sundfeldt K, Lindblom A, von Wachenfeldt Wappling A, Haanpaa M, Puistola U, Rosengren A, Anttila M et al (2011) Further evidence for the contribution of the RAD51C gene in hereditary breast and ovarian cancer susceptibility. Breast Cancer Res Treat 130(3):1003–1010
Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, Roeb W, Agnew KJ, Stray SM, Wickramanayake A et al (2011) Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci USA 108(44):18032–18037
Wong MW, Nordfors C, Mossman D, Pecenpetelovska G, Avery-Kiejda KA, Talseth-Palmer B, Bowden NA, Scott RJ (2011) BRIP1, PALB2, and RAD51C mutation analysis reveals their relative importance as genetic susceptibility factors for breast cancer. Breast Cancer Res Treat 127(3):853–859
Zheng Y, Zhang J, Hope K, Niu Q, Huo D, Olopade OI (2010) Screening RAD51C nucleotide alterations in patients with a family history of breast and ovarian cancer. Breast Cancer Res Treat 124(3):857–861
Couch FJ, Hart SN, Sharma P, Toland AE, Wang X, Miron P, Olson JE, Godwin AK, Pankratz VS, Olswold C et al (2015) Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol 33(4):304–311
Sopik V, Akbari MR, Narod SA (2015) Genetic testing for RAD51C mutations: in the clinic and community. Clin Genet 88(4):303–312
Janatova M, Soukupova J, Stribrna J, Kleiblova P, Vocka M, Boudova P, Kleibl Z, Pohlreich P (2015) Mutation Analysis of the RAD51C and RAD51D genes in high-risk ovarian cancer patients and families from the Czech Republic. Plos One 10(6):e0127711
Tavtigian SV, Deffenbaugh AM, Yin L, Judkins T, Scholl T, Samollow PB, de Silva D, Zharkikh A, Thomas A (2006) Comprehensive statistical study of 452 BRCA1 missense substitutions with classification of eight recurrent substitutions as neutral. J Med Genet 43(4):295–305
McKenzie HA, Fung C, Becker TM, Irvine M, Mann GJ, Kefford RF, Rizos H (2010) Predicting functional significance of cancer-associated p16(INK4a) mutations in CDKN2A. Hum Mutat 31(6):692–701
Tavtigian SV, Byrnes GB, Goldgar DE, Thomas A (2008) Classification of rare missense substitutions, using risk surfaces, with genetic- and molecular-epidemiology applications. Hum Mutat 29(11):1342–1354
Lohmueller KE, Sparso T, Li Q, Andersson E, Korneliussen T, Albrechtsen A, Banasik K, Grarup N, Hallgrimsdottir I, Kiil K et al (2013) Whole-exome sequencing of 2,000 Danish individuals and the role of rare coding variants in type 2 diabetes. Am J Hum Genet 93(6):1072–1086
Acknowledgments
Bettina M. Andersen, Lis Krüger, Berit B. Jensen, Aseeba Ayub, Karina Nørgaard, and Cecilia Haakansson are thanked for their help with the genetic screening of breast and/or ovarian cancer patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jønson, L., Ahlborn, L.B., Steffensen, A.Y. et al. Identification of six pathogenic RAD51C mutations via mutational screening of 1228 Danish individuals with increased risk of hereditary breast and/or ovarian cancer. Breast Cancer Res Treat 155, 215–222 (2016). https://doi.org/10.1007/s10549-015-3674-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3674-y