Abstract
Hereditary cancer predisposition gene testing allows the identification of individuals at high risk of cancer that may benefit from increased surveillance, chemoprevention, and prophylactic surgery. In order to implement clinical genetic strategies adapted to each population’s needs and intrinsic genetic characteristic, this review aims to present the current status of knowledge about the spectrum of BRCA pathogenic variants in Latin American populations. We have conducted a comprehensive review of 33 studies published between 1994 and 2015 reporting the prevalence and/or spectrum of BRCA1 (OMIM 113705) and BRCA2 (OMIM 600185) variants. The combined sample size for these studies consisted of 4835 individuals from 13 countries in Latin America and the Caribbean, as well as in Hispanics in the United States. A total of 167 unique pathogenic variants have been reported in the existing literature. In unselected breast cancer cases, the prevalence ranged from 1.2 to 27.1 %. Some countries presented a few recurrent pathogenic variants, while others were characterized by diverse, non-recurrent variants. The proportion of BRCA pathogenic variants shared between Hispanics in the United States and Latin American populations was estimated at 10.4 %. Within Latin America and the Caribbean, 8.2 % of the BRCA variants reported were present in more than one country. Countries with high prevalence of BRCA pathogenic variants may benefit from more aggressive testing strategies, while testing of recurrent variant panels might present a cost-effective solution for improving genetic testing in some, but not all, countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, breast cancer is the most common female cancer and the most common cause of female cancer-related death [1]. Breast cancer is a heterogeneous disease defined by various molecular subtypes, each one with an associated risk profile and treatment recommendations [2, 3]. Several loci of high, moderate, and low penetrance have been implicated in inherited risk of breast cancer [4, 5]. The genetic predisposition to breast cancer can be described as a continuum from a strong genetic contribution to a weaker polygenic genetic contribution with an environmental component.
Over 20 years ago, the BRCA1 and BRCA2 genes were identified as highly penetrant susceptibility genes for hereditary breast and ovarian cancers [6, 7]. Inactivating variants in those tumor suppressors account for approximately 16 % of the familial risk of breast cancer [8] and represent 5–10 % of all breast cancers [9]. Women carrying a BRCA mutation are estimated to have a cumulative cancer risk by age of 70 years of up to 87 and 54 % for breast and ovarian cancer, respectively [10–16]. They also have modest to marked increases in the lifetime risk of developing other cancer types such as colorectal, gastric, gallbladder and bile duct, malignant melanoma, pancreatic, prostate, and uterine cancers [10, 14, 16, 17]. The early identification of individuals at risk by genetic testing has been shown to be associated with increased surveillance and risk-reduction strategies ultimately leading to diagnosis of early stage tumors and improved outcomes [18, 19]. However, effective identification of BRCA1/2 mutation carriers depends on the availability and access to genetic counseling and testing of at-risk individuals.
A significant portion of populations inhabiting Central and South America as well as certain islands of the Caribbean share an official language derived from Latin, majorly Spanish or Portuguese. Despite this common heritage from the colonization era, each region of Latin America and the Caribbean (hereby referred to as Latin America) is often characterized by unique geography, culture, politics, and socioeconomic factors [20]. In the USA, Hispanics (defined by the U.S. Census Bureau a person of Cuban, Mexican, Puerto Rican, South or Central American—except for Brazil, or other Spanish culture or origin regardless of race) make up 16.3 % of the population and accounted for 56 % of the national population growth between 2000 and 2010, which makes this group the fastest growing minority in the country [21].
Despite lower incidence of breast cancer in Latin America populations when compared to Europe or the USA, the mortality-to-incidence ratio is significantly higher in the former region [21, 22]. This is in part due to poor access to cancer care and presentation at later stages [22]. For instance, it is estimated that 58 % of the breast cancers in Mexico are detected at stage III–IV [23]. In this population, the high cost associated with cancer care was attributed to late diagnosis, suggesting a need for the implementation of better prevention and detection strategies [24]. However, in several regions of Latin America, genetic testing is either not available or the associated cost is still prohibitive for a large proportion of individuals at risk [22].
There is limited information available regarding population-specific risks and very few systematic studies of the prevalence of genetic variants predisposing to breast cancer relevant to the populations of Latin America. Here we review the current knowledge about BRCA1 and BRCA2 variants in women from Latin America, the Caribbean, and Hispanics in the United States with the objective of identifying areas in need of further research and avenues for improving access to genetic testing for hereditary cancers.
The BRCA1 and BRCA2 mutation spectrum in Latin America
In recent years, increasing accessibility of next-generation sequencing (NGS) has resulted in its increased use in clinical genetic testing [25]. NGS approaches allows the cost-effective interrogation of multiple targets and has driven the expansion from BRCA1 and BRCA2 testing to the so-called ‘panel testing,’ in which a number of additional high- and moderate-penetrance hereditary cancer genes (demonstrated and suspected) are included [26]. Despite these advances, the cost of comprehensive BRCA1 and BRCA2 analysis is still prohibitive for low income individuals in these countries. Importantly, although the technical sensitivity of these assays is very high and virtually all variants can be accurately detected, our ability to clinically annotate these variants has lagged.
As a solution, amplicon-based single-site panels of recurrent Latin American BRCA pathogenic variants that would constitute the first line of screening of at-risk individuals prior to performing comprehensive BRCA analysis have been developed. Such model of tiered testing has been widely successful in the Ashkenazi Jewish population in which three common pathogenic variants explain 78.4 % of the cases [27]. The development of a clinically useful panel in Latin America will require knowledge of the pathogenic variant spectrum in each region or country and depend on the presence of recurrent variants explaining a majority of the hereditary breast and ovarian cancer cases.
To provide a comprehensive picture we have conducted a literature review of the BRCA1 and BRCA2 variants identified in Latin America (Supplementary Fig. 1). Between 1994 and August 2015, 33 publications reported BRCA variants in populations from 13 countries of Central and South America, the Caribbean, and Hispanic/Latino populations from the US. Taken together, these studies have screened a total of 4835 individuals and identified 167 different pathogenic variants (Supplementary Table 1). Table 1 summarizes the characteristics of the cohorts, the prevalence of the BRCA variants reported, and screening methods for each of the study reviewed.
The size of the cohorts ranged from 19 to 815 individuals, the majority identified through hospital or clinic-based recruitment strategies (Table 1). Several methodological factors preclude a systematic comparison across studies. There was significant variation in the inclusion criteria from unselected cases to cases selected for family history and/or personal history criteria such as age of onset and tumor molecular profile. Indirect mutation detection methods or approaches that restrict the search to panels of known mutations are expected to detect only a fraction of the variants present in the sample screened. For instance, the sensitivity of single-stranded conformation polymorphism (SSCP) ranges from 50 to 96 %, while conformation-sensitive gel electrophoresis (CSGE) and for the protein truncation test (PTT) are estimated to detect only 75 and 76 % of the BRCA1 and BRCA2 variants, respectively [28].
Early BRCA testing focused on the detection of single nucleotide variants (point mutations) and small insertions or deletions rather than large genomic rearrangements. The prevalence of such rearrangement variants has been shown to vary significantly in different populations [29]. Large genomic rearrangements may account for up to 21.4 % of the BRCA inactivating variants in high-risk patients from Latin America and the Caribbean [30]. In Hispanics from the Northern California Breast Cancer Family Registry, the BRCA1 deletion of exon 9–12 represented over 10 % of the BRCA1 pathogenic variants identified [31]. In Puerto Rico, the BRCA2 exon 1–2 deletion is one of the three most frequent pathogenic variants, accounting for 18 % of the BRCA cases [32]. Less than half of the studies reviewed reported screening for large scale rearrangements and only four reported performing comprehensive screening through Multiplex Ligation-dependent Probe Amplification (MLPA) (Table 1). As a result of these factors, the prevalence and the diversity of the BRCA pathogenic variants may be underestimated in several of the published studies.
Despite those limitations, some observations stand out. In unselected breast cancer cases from Brazil [33], Colombia [34, 35], Cuba [36], Mexico [37, 38], and Peru [39], the prevalence ranged between 1.2 and 4.9 %, which is similar to what has been reported for US non-Hispanic White populations [40]. One exception is in the Bahamas, where it is estimated that 27.1 % of breast cancer cases were carriers of a BRCA pathogenic variant, regardless of age of onset or family history of cancers [41, 42].
Currently, recommendations to offer genetic testing for hereditary cancers are based on family history and/or personal history suggestive of Hereditary Breast and Ovarian Cancer. Recently, this practice has been challenged and screening every woman as part of the routine medical examination has been proposed [43]. The high prevalence in the Bahamas provides an argument in favor of a more aggressive BRCA screening approach in such populations.
It is expected that countries for which smaller cohorts were screened show less diversity in the BRCA variants identified, not because they are less genetically diverse but rather as a consequence of the limitations associated with sample size in characterizing rare variants. This is exemplified by Brazil [33, 44–48], Mexico [37, 38, 49–52], and in US Hispanics [31, 53–57] where the number of different pathogenic variants detected ranged from 37 to 45 as a result of over 1300 individuals screened (Table 2). Variants such as 185delAG, 5382insC, and R1443X are among the 20 most frequent BRCA1 variants reported by the Breast Cancer Information Core (BIC) database (http://research.nhgri.nih.gov/bic/), and it is therefore not surprising that they are recurrent in several countries of Latin America as well. In contrast, BRCA1 A1708E was among the 10 most frequent pathogenic variants in Latin America, observed in four different countries, but is not one of the most frequent BRCA1 variants overall. This represents an example of a recurrent pathogenic variant specific to these populations. Interestingly, the ratio of BRCA1 to BRCA2 carriers also shows significant variations across Latin America. In most populations, BRCA1 variants are more frequent than BRCA2, and this holds true for most countries of Latin America. However, in Costa Rica [58], Cuba [36], Puerto Rico [32], and Uruguay [59], over 80 % of the carriers had a pathogenic variant in BRCA2. This observation has important clinical implications as the characteristics of the cancer spectrum, and tumor pathology is expected to differ according to the gene mutated [60]. For instance, BRCA1 and BRCA2 carriers show differences in both overall risk of developing breast and ovarian cancer and age distribution of risk [13]. Cancers associated with BRCA1 germline mutations are more frequently medullary of high mitotic counts, while BRCA2-associated cancers have higher score for tubule formation with lower mitotic counts [61]. Compared to sporadic breast tumors, basal subtype markers are more common in tumors arising in BRCA1 carriers [62] but not in BRCA2 carriers [63]. In contrast, BRCA2-associated tumors are more likely to be of the luminal subtype and estrogen receptor positive when compared to controls [64].
In some countries, a few recurrent variants explain the majority of cases linked to BRCA-inactivation. As a result, a PCR-based MassARRAY (Sequenom) panel of recurrent mutations, the HISPANEL, was developed in the US and validated among US Hispanic and Mexican populations. Compared with direct sequencing, this approach was estimated to have a sensitivity of 68 % but for a fraction of the cost, which is an important factor to consider in countries where resources are limited. Such an approach could be considered for Colombia where despite having identified 63 carriers in 1195 individuals screened, three recurrent variants explained over 88 % of the cases [34, 35, 65, 66]. On the other hand, this strategy may not be adequate for countries such Argentina [67], Uruguay [59], and Venezuela [68] where most recurrent pathogenic variants are not frequent. (Table 2).
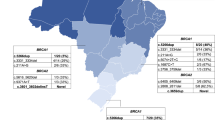
In addition, the question remains whether this panel could be transferable from one country to another. The E1308X variant is the most frequent in Puerto Rico and has been observed in the US but has not been observed in any other countries of Latin America. Within Latin America, only 8.02 % (n = 13) of the pathogenic variants reported were present in two or more countries. Caution should also be taken in studying US Hispanic to make inferences about the populations of origin of these immigrants. The BRCA2 6174delT variant was one of the most frequently observed in Latin America (except for Mexico) but has still not been reported in US Hispanics. Many of the recurrent variants found in Chile [69, 70] have not been observed in US Hispanics. Overall, of the 167 BRCA pathogenic variants identified in this literature review, only 10.4 % (n = 17) were shared between US Hispanics and Latin America. While these observations may be the result of the limited sample sizes available for some of the countries, it suggests that the Latin America immigrants established in the US might not represent the genetics of their populations of origin in their complexity. It is also a reflection of the demographics of the US Hispanic populations, where countries like Mexico and Puerto Rico are overrepresented. As illustrated by the network of interaction of recurrent BRCA variants between the Latin America countries (Fig. 1), it can be observed that the most frequent variants are more likely to be shared across countries, but a significant number of recurrent variants are found in one country only. Therefore, the available data, even though limited, suggest that the development of a low-cost Latin American screening panel that would be widely offered is unlikely as a result of the limited overlap in the spectrum of reported BRCA1 and BRCA2 variants in Latin America and US Hispanics.
The network of recurrent BRCA1 (a) and BRCA2 (b) pathogenic variants reported in Latin America, the Caribbean, and US Latinos. Recurrent variants have been reported more than once within a country and/or have been observed in more than one country. The connections between the nodes generate a complete system of interactions between variants and the countries in which they have been detected. The nodes representing the countries are mapped on the network based on the proportion of shared variants with other Latin America countries. The size of the nodes representing the variants is proportional to the total number of observations, dashed edges were used for recurrent variants that were unique to a country, and thicker edges indicate variants that are shared in more countries. The blue node colors indicate countries for which at least one study conducted comprehensive BRCA1/2 analysis by direct sequencing, and the green nodes indicate countries for which BRCA analysis was conducted using mutation panels or indirect analysis methods. Darker nodes (dark blue or dark green) indicate countries for which at least one study conducted comprehensive large rearrangement screening, while the pale node colors (pale blue or pale green) indicate countries for which no comprehensive rearrangements were assessed. The network was generated with Cytoscape 3.2.1 [96]
Population history shaped the BRCA variant spectrum in Latin America
The genetic makeup of modern populations in Latin America is a combination of pre-Colombian and colonial heritage, both in the diversity of the gene pools imported in the Americas and their subsequent admixture. Accordingly, heterogeneity in admixture patterns has been shown to vary significantly across and within the continent [71–76]. From the 15th century, the European colonial migration to the New World nearly decimated the Native American populations [77]. European founders were mainly of Spanish and Portuguese origin but also reflected the diversity of the Iberian peninsula [71]. During the Atlantic trade between 1451 and 1870, an estimated nine million slaves were brought to the Americas from the West Coast of Africa [78]. In the context of testing for hereditary cancer predisposition variants, data from the 1000 Genomes Consortium demonstrated that rare variants are more likely to be specific to a continent or country than common variants [79], suggesting that one should account for the diversity of the populations inhabiting Latin America when designing screening and prevention strategies.
Some recurrent pathogenic variants in the populations from Latin America can be associated with documented migration events. The origin of the Jewish pathogenic variant BRCA1 185delAG can be traced back to 1492 when, coinciding with the start of the colonization of the Americas, Jews were forced to convert or be expelled from the Iberian Peninsula. In the view of the long-lasting genetic admixture of Sephardic and Ashkenazi Jews, and following migration of Jews into the countries of South and North America, it is not surprising that typical founder Jewish mutations are commonly found in the modern Argentina, Peru, Brazil, Chile, and US Hispanics. In some cases, haplotype analysis has confirmed that the origin of the 185delAG in populations from Latin America was in fact the same as the Jewish founder mutation [57].
Fewer recurrent variants are traced back to the African continent, despite a significant contribution of African ancestry to the genetic pool of some of the populations of Latin America. Among the factors proposed to explain, this observation is the exceptional genetic diversity both within Africa and in the numerous ethnic groups that were brought to the Americas during the Atlantic slave trade. One exception is the BRCA1 943ins10 mutation, which is the most frequently pathogenic variant reported in African-Americans by Myriad Genetics Laboratories, representing 10 % of the 279 African-American BRCA pathogenic variants [80]. The presence of a single haplotype in families from the Ivory Coast, Florida, Southeastern US, and the Bahamas is consistent with a founder effect originating in West Africa [81].
Taken together, these examples indicate that the mutation spectrum of each Latin American population is expected to be strongly linked to their migration history. Hence, one could extrapolate that the extent of the overlap in the frequent BRCA mutations observed between countries will be, at least in part, determined by the shared events and interchanges that characterize the migration history of each geographical region. The BRCA1 deletion of exons 9–12 is a recurrent pathogenic variant observed in unrelated families of Mexican origin, which are geographically dispersed in the US [31, 82]. Haplotype analysis pointed to a founder effect of a variant that would have originated in Amerindian or Mestizo populations [82]. Other examples include a BRCA1 3450del4 identified in multiple breast and ovarian cancer patients from Brazil [45, 47], Chile [70], and Colombia [34, 35, 65, 66], which is also common in breast cancer populations from Spain [83] and Portugal [84]. In contrast, the BRCA1 5382insC founder pathogenic variant is most frequently reported in Brazil by several independent studies [33, 44, 45, 48] but has not been observed elsewhere in South America with the exception of an Ashkenazi community in Argentina [67]. This indicates that the genetic background of the Latin American populations is also shaped by events leading to unique population structures within and between countries.
Caution should be taken when making inferences about the origin of BRCA variants based solely on the co-occurrence in two populations. In the absence of haplotype analysis, one cannot distinguish whether a variant has migrated from one historically linked geographic area to another or is the result of independent mutational events. Therefore, not all carriers of the recurrent pathogenic variants or well-known founder mutations are expected to share a common ancestor. For example, BRCA2 3034del4 is a recurrent variant, which was also observed as a de novo germline mutation in a case of early onset breast cancer with no strong family history of cancer [85]. In families of Northern Europe Caucasian ancestry, this same mutation was observed in seven different countries and showed considerable haplotype diversity [86]. Haplotype analyses have identified multiple origins for several other recurrent mutations, including BRCA1 185delAG [87]. This suggests that some regions of the BRCA1 and BRCA2 genes might be prone to mutations. It is also noteworthy in light of the small proportion of studies who conduct mutation screening in the context of haplotype analysis. Finally, de novo variants may also be underreported as the majority of individuals undergoing BRCA testing are selected for having strong family history of cancer.
Cancer genetic testing awareness and access in Latin American populations
While hereditary clinical testing of Latin American and US Hispanic populations could greatly benefit from a better understanding of the mutation spectrum in the BRCA genes, social-economic aspects of genetic testing will also need to be addressed to improve access. In its policy statement on genetic and genomic testing, ASCO emphasizes the role of the informed consent process and recommends that cancer genetic susceptibility testing be conducted in the context of pre- and post-testing genetic counseling [88]. Positive outcomes associated with testing may be limited in the absence of genetic counseling and integrated clinical management structure ensuring that carriers are engaged in appropriate screening and prevention programs. Given that Hispanics in the US are 1.8 times more likely to receive an inconclusive result (variant of uncertain significance) [80], the participation of genetics professionals pre- and post-testing becomes especially important to provide support to the patients and physicians. Finally, a negative test results in the absence of a known mutation in the family may provide a false reassurance, especially when testing is not conducted in the context of extended panels of genes [89].
Studies reporting on awareness and access to genetic testing in Latin America are sparse, but the situation in the US has been more documented. In a cross-sectional analysis of 46,276 women who underwent BRCA genetic testing between 1996 and 2006, only 4.2 % were of Latin American ethnicity [80]. In a US sample of 1414 women diagnosed with breast cancer at or before 40 years of age between 2004 and 2007, Hispanic women were significantly less likely to be tested for BRCA1 and BRCA2 mutations [90]. This disparity in access to cancer genetic testing is especially alarming knowing that the Hispanic population accounts for approximately 16.3 % of the US population [21]. Genetic testing awareness has been shown to be significantly lower in Hispanics of the US when compared to non-Hispanic whites [91]. Acculturation, especially the use of the English language, strongly correlated with awareness of genetic testing for cancer in the US [92]. Once informed, participants held favorable attitudes toward risk assessment and counseling [93, 94]. In some parts of Latin America, limited resources both monetary and in the availability of trained genetic professional may impede accessibility to cancer genetic testing. Recently, BRCA1/2 telephone counseling was shown to be as effective in providing psychological support and guiding informed decisions [95]. This might contribute to improve access to genetic counseling in Latin American, especially in rural setting.
Conclusions
Therefore, for the populations of Latin America and US Hispanics to benefit from genetic-based cancer prevention options, a strategy that combines acquiring a better knowledge of the variants underlying hereditary cancers in those population and improved access to genetic testing will need to be developed. From the data available, the following recommendations emerge: (1) If panels of mutation are the only economically feasible approach, they should be developed after comprehensive analysis of a large series of samples rather than testing panels that have been developed from other populations; (2) large genomic rearrangements should be included; and (3) proper clinical infrastructure including genetic counseling should be widely available.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Ellis MJ, Perou CM (2013) The genomic landscape of breast cancer as a therapeutic roadmap. Cancer Discov 3:27–34
The Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumors. Nature 490:61–70
Walsh T, King MC (2007) Ten genes for inherited breast cancer. Cancer Cell 11:103–105
Fanale D, Amodeo V, Corsini LR, Rizzo S, Bazan V, Russo A (2012) Breast cancer genome-wide association studies: there is strength in numbers. Oncogene 31:2121–2128
Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, Bell R, Rosenthal J et al (1994) A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266:66–71
Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C, Micklem G, Barfoot R, Hamoudi R et al (1995) Identification of the breast cancer susceptibility gene BRCA2. Nature 378:789–792
Peto J, Collins N, Barfoot R, Seal S, Seal S, Warren W, Rahman N, Eatson DF, Deacon J, Stratton MR (1999) Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst 91:943–949
Claus EB, Schildkraut JM, Thompson WD, Risch NJ (1996) The genetic attributable risk of breast and ovarian cancer. Cancer 77:2318–2324
Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL (2002) Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst 94:1365–1372
Chen S, Parmigiani G (2007) Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 25:1329–1333
Easton DF, Ford D, Bishop DT (1995) Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet 56:265–271
Antoniou A, Pharoah PDP, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg Å, Pasini B, Radice P et al (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72:1117–1130
Ford D (1994) Risks of cancer in BRCA1-mutation carriers. Lancet 343:692–695
King MC, Marks JH, Mandel JB, New York Breast Cancer Study Group (2003) Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 302:643–646
Breast Cancer Linkage Consortium (1999) Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 91:1310–1316
Thompson D, Easton DF (2002) Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst 94:1358–1365
Pruthi S, Gostout BS, Lindor NM (2010) Identification and management of women with BRCA mutations or hereditary predisposition for breast and ovarian cancer. Mayo Clin Proc 85:1111–1120
Scheuer L, Kauff N, Robson M, Kelly B, Barakat R, Satagopan J, Ellis N, Hensley M, Boyd J, Brogen P, Norton L, Offit K (2002) Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol 20:1260–1268
Ferreira FH, Messina J, Rigolini J, Lopez-Calva L-F, Lugo MA, Vakis R (2013) Economic mobility and the rise of the Latin American middle class. World Bank, Washington, DC
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin 62:283–298
Goss PE, Lee BL, Badovinac-Crnjevic T, Strasser-Weippl K, Chavarri-Guerra Y, Louis JS, Villarreal-Garza C, Unger-Saldaña K, Ferreyra M, Debiasi M, Liedke PE, Touya D et al (2013) Planning cancer control in Latin America and the Caribbean. Lancet Oncol 14:391–436
Chavarri-Guerra Y, Louis JS, Liedke PE, Symecko H, Villarreal-Garza C, Mohar A, Finkelstein DM, Goss PE (2014) Access to care issues adversely affect breast cancer patients in Mexico: oncologists’ perspective. BMC Cancer 14:658
Mohar A, Bargalló E, Ramírez MT, Lara F, Beltrán-Ortega A (2009) Recursos disponibles para el tratamiento del cáncer de mama en México. Salud Publica Mex 51:s263–s269
Xuan J, Yu Y, Qing T, Guo L, Shi L (2013) Next-generation sequencing in the clinic: promises and challenges. Cancer Lett 340:284–295
Domchek SM, Bradbury A (2013) Multiplex genetic testing for cancer susceptibility: out on the high wire without a net? J Clin Oncol 31:1267–1270
Frank TS, Deffenbaugh AM, Reid JE, Hulick M, Ward BE, Lingenfelter B, Gumpper KL, Scholl T, Tavtigian SV, Pruss DR, Critchfield GC (2002) Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol 20:1480–1490
Gerhardus A, Schleberger H, Schlegelberger B, Gadzicki D (2007) Diagnostic accuracy of methods for the detection of BRCA1 and BRCA2 mutations: a systematic review. Eur J Hum Genet 15:619–627
Ewald IP, Ribeiro PL, Palmero EI, Cossio SL, Giugliani R, Ashton-Prolla P (2009) Genomic rearrangements in BRCA1 and BRCA2: a literature review. Genet Mol Biol 32:437–446
Judkins T, Rosenthal E, Arnell C, Burbidge LA, Geary W, Barrus T, Schoenberger J, Trost J, Wenstrup RJ, Roa BB (2012) Clinical significance of large rearrangements in BRCA1 and BRCA2. Cancer 118:5210–5216
Weitzel JL, Clague J, Martir-Negron A, Ogaz R, Herzog J, Ricker C, Jungbluth C, Cina C, Duncan P, Unzeitig G, Saldivar JS, Beattie M et al (2013) Prevalence and type of BRCA mutations in Hispanics undergoing genetic cancer risk assessment in the southwestern United States: a report from the Clinical Cancer Genetics Community Research Network. J Clin Oncol 31:210–216
Dutil J, Colon-Colon J, Matta JL, Sutphen R, Echenique M (2012) Identification of the prevalent BRCA1 and BRCA2 mutations in the female population of Puerto Rico. Cancer Genet 205:242–248
Gomes MCB, Costa MM, Borojevic R, Monteiro ANA, Vieira R, Koifman S, Koifman RJ, Li S, Royer R, Zhang S, Narod SA (2007) Prevalence of BRCA1 and BRCA2 mutations in breast cancer patients from Brazil. Breast Cancer Res Treat 103:349–353
Torres D, Umana A, Robledo JF (2009) Estudio de factores genéticos para cáncer de mama en Colombia. Univ Med Bogotá 50:297–301
Hernandez JE, Llacuachaqui M (2014) Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from Medellín, Colombia. Hered Cancer Clin Pract 12:11
Rodriguez RC, Esperon AA, Ropero R, Rubio MC, Rodriguez R, Ortiz RM, Anta JJL, de los Rios M, Carnesolta D, del Olivera MC, Vansam SS, Royer R et al (2008) Prevalence of BRCA1 and BRCA2 mutations in breast cancer patients from Cuba. Fam Cancer 7:275–279
Villarreal-Garza C, Alvarez-Gomez RM, Pérez-Plasencia C, Herrera LA, Herzog J, Castillo D, Mohar A, Castro C, Gallardo LN, Gallardo D, Santibáñez M, Blazer KR et al (2015) Significant clinical impact of recurrent BRCA1 and BRCA2 mutations in Mexico. Cancer 121:372–378
Torres-Mejía G, Royer R, Llacuachaqui M, Akbari MR, Giuliano AR, Martínez-Matsushita L, Angeles-Llerenas A, Ortega-Olvera C, Ziv E, Lazcano-Ponce E, Phelan CM, Narod SA (2015) Recurrent BRCA1 and BRCA2 mutations in Mexican women with breast cancer. Cancer Epidemiol Biomarkers Prev 24:498–505
Abugattas J, Llacuachaqui M, Allende YS, Velásquez AA, Velarde R, Cotrina J, Garcés M, León M, Calderón G, de la Cruz M, Mora P, Royer R et al (2014) Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from Peru. Clin Genet. doi:10.1111/cge.12505
Malone KE, Daling JR, Doody DR, Hsu L, Bernstein L, Coates RJ, Marchbanks PA, Simon MS, McDonald JA, Norman SA, Strom BL, Burkman RT et al (2006) Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res 66:8297–8308
Akbari MR, Donenberg T, Lunn J, Curling D, Turnquest T, Krill Jackson E, Zhang E, Zhang S, Narod SA, Hurley J (2014) The spectrum of BRCA1 and BRCA2 mutations in breast cancer patients in the Bahamas. Clin Genet 85:64–67
Donenberg T, Lunn J, Curling D, Turnquest T, Krill-Jackson E, Royer R, Narod SA, Hurley J (2011) A high prevalence of BRCA1 mutations among breast cancer patients from the Bahamas. Breast Cancer Res Treat 125:591–596
King MC, Levy-Lahad E, Lahad A (2014) Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA 312:1091–1092
Dufloth RM, Carvalho S, Heinrich JK, Shinzato JY, Santos CCD, Zeferino LC, Schmitt F (2005) Analysis of BRCA1 and BRCA2 mutations in Brazilian breast cancer patients with positive family history. Sao Paulo Med J 123:192–197
Esteves VF, Thuler LCS, Amêndola LC, Koifman RJ, Koifman S, Frankel PP, Vieira RJS (2009) Prevalence of BRCA1 and BRCA2 gene mutations in families with medium and high risk of breast and ovarian cancer in Brazil. Braz J Med Biol Res 42:453–457
Ewald IP, Izetti P, Vargas FR, Moreira MA (2011) Prevalence of the BRCA1 founder mutation c. 5266dup in Brazilian individuals at-risk for the hereditary breast and ovarian cancer syndrome. Hered Cancer Clin Pract 9:12
Felix GE, Abe-Sandes C, Machado-Lopes TM, Bomfim TF, Guindalini RSC, Santos VCS, Meyer L, Oliveira PC, Neiva JC, Meyer R, Romeo M, Toralles MB et al (2014) Germline mutations in BRCA1, BRCA2, CHEK2 and TP53 in patients at high-risk for HBOC: characterizing a Northeast Brazilian population. Hum Genome Var 1:14012
Silva FC, Lisboa BC, Figueiredo MC, Torrezan GT, Santos ÉM, Krepischi AC, Rossi BM, Achatz MI, Carraro DM (2014) Hereditary breast and ovarian cancer: assessment of point mutations and copy number variations in Brazilian patients. BMC Med Genet 15:55
Ruiz-Flores P, Sinilnikova OM, Badzioch M, Calderón-Garcidueñas AL, Chopin S, Fabrice O, González Guerrero JF, Szabo C, Lenoir G, Goldgar DE, Barrera-Saldaña HA (2002) BRCA1 and BRCA2 mutation analysis of early-onset and familial breast cancer cases in Mexico. Hum Mut 20:474–475
Calderón-Garcidueñas AL, Ruiz-Flores P, Cerda-Flores RM, Barrera-Saldaña HA (2005) Clinical follow up of Mexican women with early onset of breast cancer and mutations in the BRCA1 and BRCA2 genes. Salud Publica Mex 47:110–115
Vaca-Paniagua F, Alvarez-Gomez RM, Fragoso-Ontiveros V, Vidal-Millan S, Herrera LA, Cantú D, Bargallo-Rocha E, Mohar A, López-Camarillo C, Pérez-Plasencia C (2012) Full-exon pyrosequencing screening of BRCA germline mutations in Mexican women with inherited breast and ovarian cancer. PLoS ONE 7:e37432
Villarreal-Garza C, Weitzel JN, Llacuachaqui M, Sifuentes E, Magallanes-Hoyos MC, Gallardo L, Alvarez-Gomez RM, Herzog J, Castillo D, Royer R, Akbari M, Lara-Medina F et al (2015) The prevalence of BRCA1 and BRCA2 mutations among young Mexican women with triple-negative breast cancer. Breast Cancer Res Treat 150:389–394
Mullineaux LG, Castellano TM, Shaw J, Axell L, Wood ME, Diab S, Klein C, Sitarik M, Deffenbaugh AM, Graw SL (2003) Identification of germline 185delAG BRCA1 mutations in non-Jewish Americans of Spanish ancestry from the San Luis Valley. Colorado. Cancer 98:597–602
McKean-Cowdin R, Feigelson HS, Xia LY, Pearce CL, Thomas DC, Stram DO, Henderson BE (2005) BRCA1 variants in a family study of African–American and Latina women. Hum Genet 116:497–506
Vogel KJ, Atchley DP, Erlichman J, Broglio KR, Ready KJ, Valero V, Amos CI, Hortobagyi GN, Lu KH, Arun B (2007) BRCA1 and BRCA2 genetic testing in Hispanic patients: mutation prevalence and evaluation of the BRCAPRO risk assessment model. J Clin Oncol 25:4635–4641
John EM, Miron A, Gong G, Phipps AI, Felberg A, Li FP, West DW, Whittemore AS (2007) Prevalence of pathogenic BRCA1 mutation carriers in 5 US Racial/Ethnic Groups. JAMA 298:2869–2876
Weitzel JN, Lagos V, Blazer KR, Nelson R, Ricker C, Herzog J, McGuire C, Neuhausen S (2005) Prevalence of BRCA mutations and founder effect in high-risk Hispanic families. Cancer Epidemiol Biomarkers Prev 14:1666–1671
Gutiérrez Espeleta GA, Llacuachaqui M, García Jiménez L, Aguilar Herrera M, Loáiciga Vega K, Ortiz A, Royer R, Li S, Narod SA (2012) BRCA1 and BRCA2 mutations among familial breast cancer patients from Costa Rica. Clin Genet 82:484–488
Delgado L, Fernández G, Grotiuz G, Cataldi S, González A, Lluveras N, Heguaburu M, Fresco R, Lens D, Sabini G, Muse IM (2011) BRCA1 and BRCA2 germline mutations in Uruguayan breast and breast-ovarian cancer families. Identification of novel mutations and unclassified variants. Breast Cancer Res Treat 128:211–218
Hedenfalk I, Duggan D, Chen Y, Radmacher M, Bittner M, Simon R, Meltzer P, Gusterson B, Esteller M, Kallioniemi OP, Wilfond B, Borg A et al (2001) Gene-expression profiles in hereditary breast cancer. N Engl J Med 344(8):539–548
Lakhani SR, Jacquemier J, Sloane JP, Gusterson BA, Anderson TJ, van de Vijver MJ, Farid LM, Venter D, Antoniou A, Storfer-Isser A, Smyth E, Steel CM, Haites N et al (1998) Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst 90:1138–1145
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM et al (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 100:8418–8423
Lakhani SR, Reis-Filho JS, Fulford L, Penault-Llorca F, van der Vijver M, Parry S, Bishop T, Benitez J, Rivas C, Bignon YJ, Chang-Claude J, Hamann U, Cornelisse CJ et al (2005) Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res 11:5175–5180
Bane AL, Beck JC, Bleiweiss I, Buys SS, Catalano E, Daly MB, Giles G, Godwin AK, Hibshoosh H, Hopper JL, John EM, Layfield L et al (2007) BRCA2 mutation-associated breast cancers exhibit a distinguishing phenotype based on morphology and molecular profiles from tissue microarrays. Am J Surg Pathol 31:121–128
Torres D, Rashid MU, Gil F, Umana A, Ramelli G, Robledo JF, Tawil M, Torregrosa L, Briceno I, Hamann U (2007) High proportion of BRCA1/2 founder mutations in Hispanic breast/ovarian cancer families from Colombia. Breast Cancer Res Treat 103:225–232
Rodríguez AO, Llacuachaqui M, Pardo GG, Royer R, Larson G, Weitzel JN, Narod SA (2012) BRCA1 and BRCA2 mutations among ovarian cancer patients from Colombia. Gynecol Oncol 124:236–243
Solano AR, Aceto GM, Delettieres D, Veschi S, Neuman MI, Alonso E, Chialina S, Chacón RD, Renato M-C, Podestá EJ (2012) BRCA1 and BRCA2 analysis of Argentinean breast/ovarian cancer patients selected for age and family history highlights a role for novel mutations of putative south-American origin. Springerplus 1:20
Lara K, Consigliere N, Pérez J, Porco A (2012) BRCA1 and BRCA2 mutations in breast cancer patients from Venezuela. Biol Res 45:117–130
Gallardo M, Silva A, Rubio L, Alvarez C, Torrealba C, Salinas M, Tapia T, Faundez P, Palma L, Riccio ME, Paredes H, Rodriguez M et al (2006) Incidence of BRCA1 and BRCA2 mutations in 54 Chilean families with breast/ovarian cancer, genotype–phenotype correlations. Breast Cancer Res Treat 95:81–87
Gonzalez-Hormazabal P, Gutierrez-Enriquez S, Gaete D, Reyes JM, Peralta O, Waugh E, Gomez F, Margarit S, Bravo T, Blanco R, Diez O, Jara L (2011) Spectrum of BRCA1/2 point mutations and genomic rearrangements in high-risk breast/ovarian cancer Chilean families. Breast Cancer Res Treat 126:705–716
Salary K (2011) Gonzalez Burchard E. Latino populations: a unique opportunity for the study of race, genetics, and social environment in epidemiological research. Paediatr Perinat Epidemiol 95:2161–2168
Salzano FM, Sans M (2013) Interethnic admixture and the evolution of Latin American populations. Genet Mol Biol 37:151–170
Benn Torres J, Bonilla C, Robbins CM, Waterman L, Moses TY, Hernandez W, Santos ER, Bennett F, Aiken W, Tullock T, Coard K, Hennis A et al (2008) Admixture and population stratification in African Caribbean populations. Ann Hum Genet 72:90–98
Avena S, Via M, Ziv E, Pérez-Stable EJ, Gignoux CR, Dejean C, Huntsman S, Torres-Mejía G, Dutil J, Matta JL, Beckman K, Burchard EG et al (2012) Heterogeneity in genetic admixture across different regions of Argentina. PLoS ONE 7:e34695
Via M, Gignoux CR, Roth LA, Fejerman L, Galanter J, Choudhry S, Toro-Labrador G, Viera-Vera J, Oleksyk TK, Beckman K, Ziv E, Risch N et al (2011) History shaped the geographic distribution of genomic admixture on the island of Puerto Rico. PLoS ONE 6:e16513
Wang S, Ray N, Rojas W, Parra MV, Bedoya G, Gallo C, Poletti G, Mazzotti G, Hill K, Hurtado AM, Camrena B, Nicolini H et al (2008) Geographic patterns of genome admixture in Latin American Mestizos. PLoS Genet 4:e1000037
Burkholder MA, Johson LL (2014) Colonial Latin America, 9th edn. Oxford University Press, Oxford, p 432p
Salzano FM, Bortolini MC (2001) The evolution and genetics of Latin American populations, 1st edn. Cambridge University Press, Cambridge
Genomes Project Consortium (2012) An integrated map of genetic variation from 1092 human genomes. Nature 491:56–65
Hall MJ, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C, Wenstrup RJ, Ward BE, Scholl TA, Noll WW (2009) BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer 115:2222–2233
Mefford HC, Baumbach L, Panguluri RC, Whitfield-Broome C, Szabo C, Smith S, King MC, Dunston G, Stoppa-Lyonnet D, Arena F (1999) Evidence for a BRCA1 founder mutation in families of West African ancestry. Am J Hum Genet 65:575–578
Weitzel JN, Lagos VI, Herzog JS, Judkins T, Hendrickson B, Ho JS, Ricker CN, Lowstuter KJ, Blazer KR, Tomlinson G, Scholl T (2007) Evidence for common ancestral origin of a recurring BRCA1 genomic rearrangement identified in high-risk Hispanic families. Cancer Epidemiol Biomarkers Prev 16:1615–1620
Blesa JR, García JA, Ochoa E (2000) Frequency of germ-line BRCA 1 mutations among Spanish families from a Mediterranean area. Hum Mut 15:381–382
Peixoto A, Salgueiro N, Santos C, Varzim G, Rocha P, Soares MJ, Pereira D, Rodrigues H, Bento MJ, Fráguas A, Moura G, Regateiro F et al (2006) BRCA1 and BRCA2 germline mutational spectrum and evidence for genetic anticipation in Portuguese breast/ovarian cancer families. Fam Cancer 5:379–387
Van Der Luijt RB, van Zon PH, Jansen RP, van der Sijs-Bos CJ, Wárlám-Rodenhuis CC, Ausems MG (2001) De novo recurrent germline mutation of the BRCA2 gene in a patient with early onset breast cancer. J Med Genet 38:102–105
Neuhausen SL, Godwin AK, Gershoni-Baruch R, Schubert E, Garber J, Stoppa-Lyonnet D, Olah E, Csokay B, Serova O, Lalloo F, Osorio A, Stratton M et al (1998) Haplotype and phenotype analysis of nine recurrent BRCA2 mutations in 111 families: results of an international study. Am J Hum Genet 62:1381–1388
Neuhausen SL, Mazoyer S, Friedman L, Stratton M, Offit K, Caligo A, Tomlinson G, Cannon-Albright L, Bishop T, Kelsell D, Solomon E, Weber B et al (1996) Haplotype and phenotype analysis of six recurrent BRCA1 mutations in 61 families: results of an international study. Am J Hum Genet 58:271–280
Robson ME, Bradbury AR, Arun B, Domchek SM, Ford JM, Hampel HL, Lipkin SM, Syngal S, Wollins DS, Lindor NM (2015) American Society of Clinical Oncology Policy Statement Update: genetic and genomic testing for cancer susceptibility. J Clin Oncol JCO.2015.63.0996
Yurgelun MB, Hiller E, Garber JE (2015) Population-Wide Screening for Germline BRCA1 and BRCA2 Mutations: too much of a good thing? J Clin Oncol 33:3092–3095
Levy DE, Byfield SD, Comstock CB, Garber JE, Syngal S, Crown WH, Shields AE (2011) Underutilization of BRCA1/2 testing to guide breast cancer treatment: black and hispanic women particularly at risk. Genet Med 13:349–355
Wideroff L, Vadaparampil ST, Breen N, Croyle RT, Freedman AN (2003) Awareness of genetic testing for increased cancer risk in the year 2000 National Health Interview Survey. Community Genet 6:147–156
Vadaparampil ST, Wideroff L, Breen N, Trapido E (2006) The impact of acculturation on awareness of genetic testing for increased cancer risk among Hispanics in the year 2000 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev 15:618–623
Vadaparampil ST, Quinn GP, Dutil J, Puig M, Malo TL, McIntyre J, Perales R, August EM, Closser Z (2011) A pilot study of knowledge and interest of genetic counseling and testing for hereditary breast and ovarian cancer syndrome among Puerto Rican women. J Commun Genet 2:211–221
Kinney AY, Gammon A, Coxworth J, Simonsen SE, Arce-Laretta M (2010) Exploring attitudes, beliefs, and communication preferences of Latino community members regarding BRCA1/2 mutation testing and preventive strategies. Genet Med 12:105–115
Kinney AY, Butler KM, Schwartz MD, Mandelblatt JS, Boucher KM, Pappas LM, Gammon A, Kohlmann W, Edwards SL, Stroup AM, Buys SS, Flores KG et al (2014) Expanding access to BRCA1/2 genetic counseling with telephone delivery: a cluster randomized trial. J Natl Cancer Inst 106:dju328
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Acknowledgments
This work was supported by NIH Awards U54 CA163071, U54 CA163068, U01 CA116167, and by awards from the Florida Breast Cancer Foundation and from the Moffitt Foundation. H.J. Diaz-Zabala received funding through the RISE Grant R25GM082406 from the National Institute of General Medical Sciences of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
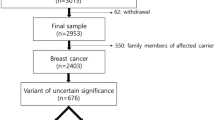
Protocol for the identification and selection of the studies to be included. Pubmed database was searched for combination of the following MeSH terms: “Genes, BRCA1” or “Genes, BRCA2” and “South America” or “Central America” or “Caribbean Region” or “Hispanic Americans” or “Mexico” and “Mutation” or “Hereditary Breast and Ovarian Cancer Syndrome”. Please note that Mexico was searched separately as it is not listed as a sub-category of “Central America” MeSH term. A total of 48 studies were identified through that search, out of which 21 met the eligibility criteria. Reasons for excluding a study included 1) no mutations identified in the sample analyzed (n = 4), sample analyzed is the same as another study already included (n = 2), gene analyzed is other than BRCA1 or BRCA2 (n = 1), full text not available online (n = 1), study population is not from Latin America, Brazil or US Hispanic (n = 1), study does not conduct mutation analysis of BRCA1 and BRCA2 (n = 10). The literature cited from selected papers was systematically searched and additional eligible studies were identified (n = 11). A total of 33 eligible studies were included in the analysis. Supplementary material 1 (JPEG 83 kb)
Supplementary Table 1
Nomenclature of the pathogenic variants identified in US Hispanics, Latin America and the Caribbean. Designations from the Breast Cancer Information Core (BIC, https://research.nhgri.nih.gov/projects/bic/Member/index.shtml), Human Genome Variation Society (HGVS, http://www.hgvs.org/), the single nucleotide polymorphisms database (dbSNP, http://www.ncbi.nlm.nih.gov/projects/SNP/) and the Breast ExAC Exome Aggregation Consortium (http://exac.broadinstitute.org/) are provided. The total number of times and the countries where each mutation was reported is listed for each variant. NR not reported, N/a not applicable. Supplementary material 2 (XLSX 27 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dutil, J., Golubeva, V.A., Pacheco-Torres, A.L. et al. The spectrum of BRCA1 and BRCA2 alleles in Latin America and the Caribbean: a clinical perspective. Breast Cancer Res Treat 154, 441–453 (2015). https://doi.org/10.1007/s10549-015-3629-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3629-3