Abstract
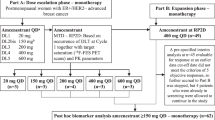
Fulvestrant, which degrades ER, is used after AI failure in metastatic breast cancer but resistance develops quickly. We hypothesized that using everolimus to inhibit mTOR, a key signaling pathway in endocrine resistance, may delay fulvestrant resistance in patients and thus improve its efficacy. We conducted a phase II trial of combined fulvestrant and everolimus in postmenopausal women with disease progression or relapse after an AI. Primary endpoint was time to progression (TTP) and secondary endpoints included objective response rate, clinical benefit rate (CBR), safety, and biomarker correlates. Tumor blocks were collected and biopsy of accessible tumor was done for future biomarker analysis. Of 33 patients enrolled two were ruled ineligible after enrollment and were excluded from study analysis, for a total of 31 evaluable patients. Median age was 54 years (range 45–85). Prior therapy included tamoxifen (81 %), chemotherapy (71 %), with 26 % of patients having received 3 or more endocrine agents. Median TTP was 7.4 months (95 % CI 1.9–12.1) with an objective response rate of 13 % and CBR of 49 %. Of particular note, 32 % of patients exhibited de novo resistance to study treatment with disease progression as their best response. Most common adverse events (AEs) were elevated AST (87 %) and ALT (77 %), anemia (74 %), hyperglycemia (71 %), and hypercholesterolemia (68 %). Prominent clinical toxicities were mucositis (58 %), weight loss (48 %), and rash (42 %). Most AEs were grade 1 or 2 and largely reversible with infrequent need for everolimus dose reduction. To conclude, everolimus plus fulvestrant is effective after AI failure in heavily pretreated metastatic ER-positive breast cancer and has manageable toxicity. Further study of this combination is warranted in randomized studies. Since not all patients experience benefit, and in view of potential toxicities, biomarker examination is critical to help select patients most likely to benefit from this strategy in future studies.



Similar content being viewed by others
References
Bonneterre J, Buzdar A, Nabholtz JM, Robertson JF, Thurlimann B, von Euler M, Sahmoud T, Webster A, Steinberg M (2001) Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer 92(9):2247–2258. doi:10.1002/1097-0142(20011101)92:9<2247:AID-CNCR1570>3.0.CO;2-Y
Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Janicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Lassus M, Verbeek JA, Staffler B, Chaudri-Ross HA, Dugan M (2001) Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 19(10):2596–2606
Mauri D, Pavlidis N, Polyzos NP, Ioannidis JPA (2006) Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst 98(18):1285–1291. doi:10.1093/jnci/djj357
Osborne C, Wakeling A, Nicholson R (2004) Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer 90(Suppl 1):S2–S6
Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, Fein L, Romieu G, Buzdar A, Robertson JF, Brufsky A, Possinger K, Rennie P, Sapunar F, Lowe E, Piccart M (2008) Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol 26(10):1664–1670. doi:10.1200/JCO.2007.13.5822
Massarweh S, Schiff R (2006) Resistance to endocrine therapy in breast cancer: exploiting estrogen receptor/growth factor signaling crosstalk. Endocr Relat Cancer 13(Supplement 1):S15–S24. doi:10.1677/erc.1.01273
Leary A, Sirohi B, Johnston S (2007) Clinical trials update: endocrine and biological therapy combinations in the treatment of breast cancer. Breast Cancer Res 9(5):112
Sabnis G, Goloubeva O, Jelovac D, Schayowitz A, Brodie A (2007) Inhibition of the phosphatidylinositol 3-kinase/Akt pathway improves response of long-term estrogen-deprived breast cancer xenografts to antiestrogens. Clin Cancer Res 13(9):2751–2757. doi:10.1158/1078-0432.ccr-06-2466
Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura A, Lawrence J Jr, Berstein L, Yue W (2005) Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity. Endocr Relat Cancer 12(Supplement 1):S61–S73. doi:10.1677/erc.1.01018
Sun M, Paciga JE, Feldman RI, Yuan Z, Coppola D, Lu YY, Shelley SA, Nicosia SV, Cheng JQ (2001) Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Res 61(16):5985–5991
Beeram M, Tan QT, Tekmal RR, Russell D, Middleton A, DeGraffenried LA (2007) Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann Oncol 18(8):1323–1328. doi:10.1093/annonc/mdm170
Treeck O, Wackwitz B, Haus U, Ortmann O (2006) Effects of a combined treatment with mTOR inhibitor RAD001 and tamoxifen in vitro on growth and apoptosis of human cancer cells. Gynecol Oncol 102(2):292–299
Miller TW, Balko JM, Arteaga CL (2011) Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol 29(33):4452–4461. doi:10.1200/jco.2010.34.4879
Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70. doi:10.1038/nature11412
Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo W-L, Davies M, Carey M, Hu Z, Guan Y, Sahin A, Symmans WF, Pusztai L, Nolden LK, Horlings H, Berns K, Hung M-C, van de Vijver MJ, Valero V, Gray JW, Bernards R, Mills GB, Hennessy BT (2008) An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 68(15):6084–6091. doi:10.1158/0008-5472.can-07-6854
Bachelot T (2012) Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO Study. J Clin Oncol 30:2718–2724
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366(6):520–529. doi:10.1056/NEJMoa1109653
Hyams DM, Chan A, de Oliveira C, Snyder R, Vinholes J, Audeh MW, Alencar VM, Lombard J, Mookerjee B, Xu J, Brown K, Klein P (2013) Cediranib in combination with fulvestrant in hormone-sensitive metastatic breast cancer: a randomized Phase II study. Invest New Drugs 31(5):1345–1354. doi:10.1007/s10637-013-9991-2
Tan WW, Dueck AC, Flynn P, Steen P, Anderson D, Rowland K, Northfelt D, Perez EA (2013) N0539 phase II trial of fulvestrant and bevacizumab in patients with metastatic breast cancer previously treated with an aromatase inhibitor: a North Central Cancer Treatment Group (now Alliance) trial. Ann Oncol 24(10):2548–2554
Robertson JF, Ferrero JM, Bourgeois H, Kennecke H, de Boer RH, Jacot W, McGreivy J, Suzuki S, Zhu M, McCaffery I, Loh E, Gansert JL, Kaufman PA (2013) Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol 14(3):228–235. doi:10.1016/S1470-2045(13)70026-3
Burstein HJ, Barry WT, Cirrincione C, Chew HK, Tolaney S, Lake D, Pluard T, Blackwell K, Winer EP, Hudis CA (2010) Fulvestrant with or without lapatinib as therapy for hormone receptor positive advanced breast cancer: a double-blinded, placebo-controlled, randomized phase III study. Cancer Res 70(24 Suppl 2):Abstr nr PD-05-01. doi:10.1158/0008-5472.SABCS10-PD05-01
Carlson RW, O’Neill A, Vidaurre T, Gomez HL, Badve SS, Sledge GW (2012) A randomized trial of combination anastrozole plus gefitinib and of combination fulvestrant plus gefitinib in the treatment of postmenopausal women with hormone receptor positive metastatic breast cancer. Breast Cancer Res Treat 133(3):1049–1056. doi:10.1007/s10549-012-1997-5
Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SG, McCue BL, Wakeling AE, McClelland RA, Manning DL, Nicholson RI (1995) Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst 87(10):746–750
Howell A, Robertson JF, Abram P, Lichinitser MR, Elledge R, Bajetta E, Watanabe T, Morris C, Webster A, Dimery I, Osborne CK (2004) Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol 22(9):1605–1613. doi:10.1200/JCO.2004.02.112
Howell A, Robertson JF, Quaresma Albano J, Aschermannova A, Mauriac L, Kleeberg UR, Vergote I, Erikstein B, Webster A, Morris C (2002) Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol 20(16):3396–3403
Osborne CK, Pippen J, Jones SE, Parker LM, Ellis M, Come S, Gertler SZ, May JT, Burton G, Dimery I, Webster A, Morris C, Elledge R, Buzdar A (2002) Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol 20(16):3386–3395
Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, Verhoeven D, Pedrini JL, Smirnova I, Lichinitser MR, Pendergrass K, Garnett S, Lindemann JPO, Sapunar F, Martin M (2010) Results of the CONFIRM phase III trial comparing fulvestrant 250 mg With fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 28(30):4594–4600. doi:10.1200/jco.2010.28.8415
Johnston SR, Kilburn LS, Ellis P, Dodwell D, Cameron D, Hayward L, Im YH, Braybrooke JP, Brunt AM, Cheung KL, Jyothirmayi R, Robinson A, Wardley AM, Wheatley D, Howell A, Coombes G, Sergenson N, Sin HJ, Folkerd E, Dowsett M, Bliss JM (2013) Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol 14(10):989–998. doi:10.1016/S1470-2045(13)70322-X
Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR, Lew DL, Hayes DF, Gralow JR, Livingston RB, Hortobagyi GN (2012) Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 367(5):435–444. doi:10.1056/NEJMoa1201622
Bergh J, Jönsson P-E, Lidbrink EK, Trudeau M, Eiermann W, Brattström D, Lindemann JPO, Wiklund F, Henriksson R (2012) FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 30(16):1919–1925. doi:10.1200/jco.2011.38.1095
Wolff AC, Lazar AA, Bondarenko I, Garin AM, Brincat S, Chow L, Sun Y, Neskovic-Konstantinovic Z, Guimaraes RC, Fumoleau P, Chan A, Hachemi S, Strahs A, Cincotta M, Berkenblit A, Krygowski M, Kang LL, Moore L, Hayes DF (2012) Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol. doi:10.1200/jco.2011.38.3331
Busaidy NL, Farooki A, Dowlati A, Perentesis JP, Dancey JE, Doyle LA, Brell JM, Siu LL (2012) Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J Clin Oncol 30(23):2919–2928. doi:10.1200/JCO.2011.39.7356
Acknowledgments
This work was supported by a grant from Novartis pharmaceuticals (S. M.). Presented in part at the 35th San Antonio Breast Cancer Symposium in 2012 (Cancer Res, December 15, 2012; 72(24 Supplement): P2-14-05, and at the 2013 American Society of Clinical Oncology Meeting, Chicago (J Clin Oncol 31, 2013, suppl; abstr 541). Registered under ClinicalTrials.gov number NCT00570921 and Investigational New Drug Application (IND) #79,103 (IND holder, S. M.).
Conflict of interests
S.M. receives research funding from Novartis and Onyx–Bayer, and honoraria/consultation fees from Novartis. All remaining authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Massarweh, S., Romond, E., Black, E.P. et al. A phase II study of combined fulvestrant and everolimus in patients with metastatic estrogen receptor (ER)-positive breast cancer after aromatase inhibitor (AI) failure. Breast Cancer Res Treat 143, 325–332 (2014). https://doi.org/10.1007/s10549-013-2810-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2810-9