Abstract
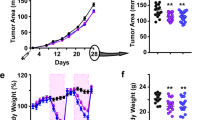
Previously we reported that intermittent calorie restriction (ICR) provided greater prevention of mammary tumors (MTs) than chronic calorie restriction (CCR). Here the impact of increased fat intake during refeeding in an ICR protocol was evaluated. MMTV-TGF-α female mice were assigned to one of three groups: ad libitum (AL) fed (n = 45) with free access to a moderately high fat diet (22 % fat calories); ICR (n = 45) 50 % calorie restricted for 3-week intervals followed by 3 weeks of 100 % of AL intake; and CCR (n = 45) fed 75 % of AL mice, matching each 6-week cycle of ICR mice. ICR mice were further designated as ICR-Restricted or ICR-Refed for data obtained during these intervals. All mice consumed the same absolute amount of dietary fat. Mice were followed to assess MT incidence, body weight and serum IGF-1, IGFBP3, leptin and adiponectin levels until 79 (end of final 3-week restriction) or 82 (end of final 3-weeks refeeding) weeks of age. Age of MT detection was significantly extended for CCR (74 weeks) and ICR (82 weeks) mice, compared to 57.5 weeks for AL mice. MT incidence for AL, ICR and CCR mice was 66.7, 4.4, and 52.3 %, respectively. Mammary and fat pad weights were reduced significantly following 50 % calorie restriction in ICR-Restricted mice compared to AL, CCR and ICR-Refed mice. IGF-1 and leptin levels also tended to be reduced in ICR-Restricted mice over the course of the study while adiponectin was not compared to AL, CCR, and ICR-Refed mice. The adiponectin:leptin ratio was consistently higher following 50 % restriction in ICR-Restricted mice. There was no relationship of IGF-1, leptin, or adiponectin with the presence of MTs in any groups. Thus the manner in which calories are restricted impacts the protective effect of calorie restriction independently of high fat intake.







Similar content being viewed by others
Abbreviations
- ICR:
-
Intermittent calorie restriction
- CCR:
-
Chronic caloric restriction
- AL:
-
ad libitum
- IGF-1:
-
Insulin-like growth factor-1
- IGFBP-3:
-
Insulin-like growth factor binding protein-3
- MMTV-TGF-α:
-
Mouse mammary tumor virus transforming growth factor-α
- MT:
-
Mammary tumor
References
Harvie M, Howell A (2006) Energy balance adiposity and breast cancer—energy restriction strategies for breast cancer prevention. Obes Rev 7:33–47
Ruggeri BA, Klurfeld DM, Kritchevsky D, Furlanetto RW (1989) Caloric restriction and 7,12-dimethylbenz(a)anthracene-induced mammary tumor growth in rats: alterations in circulating insulin, insulin-like growth factors I and II, and epidermal growth factor. Cancer Res 49:4130–4134
Klurfeld DM, Welch CB, Davis MJ, Kritchevsky D (1989) Determination of degree of energy restriction necessary to reduce DMBA-induced mammary tumorigenesis in rats during the promotion phase. J Nutr 119:286–291
Klurfeld DM, Lloyd LM, Welch CB et al (1991) Reduction of enhanced mammary carcinogenesis in LA/N-cp (corpulent) rats by energy restriction. Proc Soc Exp Biol Med 196:381–384
Fernandes G, Chandrasekar B, Troyer DA et al (1995) Dietary lipids and calorie restriction effect mammary tumor incidence and gene expression in mouse mammary tumor virus/v-Ha-ras transgenic mice. Proc Natl Acad Sci USA 92:6494–6498
Zhu Z, Haegele AD, Thompson HJ (1997) Effect of caloric restriction on pre-malignant and malignant stages of mammary carcinogenesis. Carcinogenesis 18:1007–1012
Dirx MJM, Zeegers MPA, Dagnelie PC et al (2003) Energy restriction and the risk of spontaneous mammary tumors in mice: a meta-analysis. Int J Cancer 106:766–770
Bunk B, Zhu P, Klinga K et al (1992) Influence of reducing luxury calories in the treatment of experimental mammary carcinoma. Br J Cancer 65:845–851
Beth M, Berger MR, Aksoy M, Schmahl D (1987) Comparison between the effects of dietary fat level and calorie intake on methlynitrosourea-induced mammary carcinogenesis in female SD rats. Int J Cancer 39:737–744
Klurfeld DM, Welch CB, Lloyd LM, Kritchevsky D (1989) Inhibition of DMBA-induced mammary tumorigenesis by caloric restriction in rats fed high-fat diets. Int J Cancer 43:922–925
Welsch CW, House JL, Herr BL et al (1990) Enhancement of mammary carcinogenesis by high levels of dietary fat: a phenomenon dependent on ad libitum feeding. J Natl Cancer Inst 82:1615–1620
Boissonneault GA, Elson CE, Pariza MW (1986) Net energy effects of dietary fat on chemically induced mammary carcinogenesis in F344 rats. J Natl Cancer Inst 76:335–338
Welsch CW (1992) Relationship between dietary fat and experimental mammary tumorigenesis: a review and critique. Cancer Res 52:2040s–2048s
Cleary MP, Grande JP, Maihle NJ (2004) Effect of a high fat diet on body weight and mammary tumor latency in MMTV-TGF-α mice. Int J Obes 28:956–962
Engelman RW, Day NK, Good RA (1994) Calorie intake during mammary development influences cancer risk: lasting inhibition of C3H/HeOu mammary tumorigenesis by peripubertal calorie restriction. Cancer Res 54:5724–5730
Chen R-F, Good RA, Engelman RW et al (1990) Suppression of mouse mammary tumor proviral DNA and protooncogene expression: association with nutritional regulation of mammary tumor development. Proc Natl Acad Sci USA 87:2385–2389
Carlson AJ, Hoelzel F (1946) Apparent prolongation of the life span of rats by intermittent fasting. J Nutr 31:363–375
Harris SR, Brix AE, Broderson JR, Bunce OR (1995) Chronic energy restriction versus energy cycling and mammary tumor promotion. Proc Soc Exp Biol Med 209:231–236
Tagliaferro AR, Ronan AM, Meeker LD et al (1996) Cyclic food restriction alters substrate utilization and abolishes protection from mammary carcinogenesis in female rats. J Nutr 126:1398–1405
Cleary MP, Jacobson MK, Phillips FC et al (2002) Weight-cycling decreases incidence and increases latency of mammary tumor development to a greater extent than does chronic restriction in mouse mammary tumor virus-transforming growth factor-α female mice. Cancer Epidemiol Biomarkers Prev 11:836–843
Cleary MP, Hu X, Grossmann ME et al (2007) Prevention of mammary tumorigenesis by intermittent caloric restriction, does caloric intake during refeeding modulate the response? Exp Biol Med. 232:70–80
Rogozina OP, Bonorden MJL, Grande JP, Cleary MP (2009) Serum insulin like growth factor-I and mammary tumor development in ad libitum-fed, chronic calorie-restricted and intermittent calorie-restricted MMTV-TGF-α mice. Cancer Prev Res 2:712–719
Pape-Ansorge KA, Grande JP, Christensen TA et al (2002) Effect of moderate caloric restriction and/or weight-cycling on mammary tumor incidence and latency in MMTV-neu female mice. Nutr Cancer 44:161–168
Renehan AG, Roberts DL, Dive C (2008) Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem 114:71–83
Vona-Davis L, Rose DP (2007) Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 14:189–206
Barb D, Pazaitou-Panayiotou K, Mantzoros CS (2006) Adiponectin: a link between obesity and cancer. Expert Opin Investig Drugs 15:917–933
Hu X, Juneja SC, Maihle NJ, Cleary MP (2002) Leptin- a growth factor for normal and malignant breast cells and normal mammary gland development. J Natl Cancer Inst 94:1704–1711
Laud K, Gourdou I, Pessemesse L et al (2002) Identification of leptin receptors in human breast cancer: functional activity in the T47-D breast cancer cell line. Mol Cell Endocrinol 188:219–226
Dieudonne M-N, Machinal-Quelin F, Serazin-Leroy V et al (2002) Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun 293:622–628
Ray A, Nkhata KJ, Cleary MP (2007) Effects of leptin on human breast cancer cell lines in relationship to estrogen receptor and HER2 status. Int J Oncol 30:1499–1509
Garofalo C, Sisci D, Surmacz E (2004) Leptin interferes with the effects of the antiestrogen ICI 182.780 in MCF-7 breast cancer cell lines. Clin Cancer Res 10:6466–6475
Dieudonne M-N, Bussiere M, Dos Santos E et al (2006) Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun 345:271–279
Grossmann ME, Nkhata KJ, Mizuno NK et al (2008) Effects of adiponectin on breast cancer cell growth and signaling. Br J Cancer 98:370–378
Takahata C, Miyoshi Y, Irahara N et al (2007) Demonstration of adiponectin receptors 1 and 2 mRNA expression in human breast cancer cells. Cancer Lett 250:229–236
Körner A, Pazaitou-Panayiotou K, Kelesidis T et al (2007) Total and high-molecular-weight adiponectin in breast cancer: in vitro and in vivo studies. J Clin Endocrinol Metab 92:1041–1048
Chen D-C, Chung Y-F, Yeh Y-T et al (2006) Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett 237:109–114
Mantzoros C, Petridou E, Dessypris N et al (2004) Adiponectin and breast cancer risk. J Clin Endocrinol Metab 89:1102–1107
Grossmann ME, Ray A, Dogan S et al (2008) Balance of adiponectin and leptin in relationship to breast cancer cell growth. Cell Res 18:1154–1156
Rogozina OP, Bonorden MJL, Seppanen C et al (2011) Effect of chronic and intermittent calorie restriction on serum adiponectin and leptin and mammary tumorigenesis. Cancer Prev Res 4:568–581
Dogan S, Rogozina OP, Loshkin A et al (2010) Effects of chronic vs intermittent calorie restriction on mammary tumor incidence and serum adiponecin and leptin levels in MMTV-TGF-α mice at different ages. Oncol Lett 1:167–176
Shankaraiah K, Halberg F, Yunis E, Watson LM (1984) Alternate-day feeding alters the circadian system, reduces breast cancer incidence and prolongs life. In: Halberg F, Reale L, Tarquini B (eds) Proceedings of II international symposium on chronobiologic approach to social medicine. Instituto Itaniano di Medicina Sociale, Rome, pp 633–648
Shao R, Dao ML, Day NK, Good RA (1990) Dietary manipulation of mammary tumor development in adult C3H/Bi mice. Proc Soc Exp Biol Med 193:313–317
Buison AM, Pellizzon MA, Brogan KE et al (2005) Weight cycling did not increase tumor incidence in high fat-fed rats treated with a low-dose 7,12-dimethylbenzyl(1)anthracene. Nutr Res. 25:1097–1108
Bonorden MJL, Rogozina OP, Kluczny CM et al (2009) Intermittent caloric restriction delays tumor detection and increases survival in TRAMP mice. Nutr Cancer 61:265–275
Bonorden MJL, Rogozina OP, Kluczny CM et al (2009) Cross-sectional analyses of intermittent versus chronic caloric restriction in the TRAMP mouse. Prostate 69:317–326
Buschemeyer WC III, Klink JC, Mavropoulos JC et al (2010) Effect of intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate 70:1037–1043
Descamps O, Riondel J, Ducros V, Rousell A-M (2005) Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: effect of alternate-day fasting. Mech Age Dev 126:1185–1191
Berrigan D, Perkins SN, Haines DC, Hursting SD (2002) Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis 23:817–822
Trentham-Dietz A, Newcomb PA, Egan KM et al (2000) Weight change and risk of postmenopausal breast cancer (United States). Cancer Causes Control 11:533–542
Mellemkjaer L, Emborg C, Gridley G et al (2001) Anorexia nervosa and cancer risk. Cancer Causes Control 12:173–177
Michels KB, Ekbom A (2004) Caloric restriction and incidence of breast cancer. JAMA 291:1226–1230
Harvie MN, Pegington M, Mattson MP et al (2011) The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes 35:714–727
Renehan AG, Zwahlen M, Minder C et al (2004) Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 363:1346–1353
Peyrat JP, Bonneterre J, Hecquet B et al (1993) Plasma insulin-like growth factor-1 (IGF-1) concentrations in human breast cancer. Eur J Cancer 29A:492–497
Yu H, Jin F, Shu X-O et al (2002) Insulin-like growth factors and breast cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev 11:705–712
de Ostrovich KK, Lambertz I, Colgy JKL et al (2008) Paracrine overexpression of insulin-like growth factor-1 enhances mammary tumorigenesis in vivo. Am J Pathol 173:824–834
Hadsell DL, Bonnette SG (2000) IGF and insulin action in the mammary gland: lessons from transgenic and knockout models. J Mammary Gland Biol Neoplasia 5:19–30
Dunn SE, Kari FW, French JE et al (1997) Dietary restriction reduces insulin-like growth-like factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res 57:4667–4672
Powolny AA, Wang S, Carlton PS et al (2008) Interrelationships between dietary restriction, the IGF-I axiz, and expression of vascular endothelial growth factor by prostate adenocarcinomas in rats. Mol Carcinog 47:458–465
Cleary MP, Ray A, Rogozina OP et al (2009) Targeting the adiponectin-leptin ratio for prevention of postmenopausal breast cancer. Front Biosci 1:329–357
Petridou E, Mantzoros C, Dessypris N et al (2003) Plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J Clin Endocrinol Metab 88:993–997
Tworoger SS, Eliassen AH, Kelesidis T et al (2007) Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab 92:1510–1516
Nkhata KJ, Ray A, Schuster TF et al (2009) Effects of adiponectin and leptin co-treatment on human breast cancer cell growth. Oncol Rep 21:1611–1619
Acknowledgments
This research was supported by NIH-CA 101858 and the Hormel Foundation.
Conflict of interest
We declare that we do not have a financial relationship with the organizations that sponsored the research.
Ethical standards
All procedures with mice were performed under the guidelines and with approval of the University of Minnesota Institutional Animal Care and Use Committee. The University of Minnesota is an AAALAC accredited institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rogozina, O.P., Nkhata, K.J., Nagle, E.J. et al. The protective effect of intermittent calorie restriction on mammary tumorigenesis is not compromised by consumption of a high fat diet during refeeding. Breast Cancer Res Treat 138, 395–406 (2013). https://doi.org/10.1007/s10549-013-2464-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2464-7