Abstract
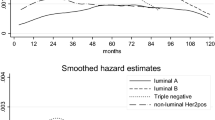
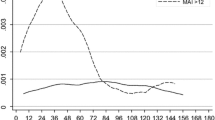
Although pathologic complete response after neoadjuvant systemic chemotherapy (NST) is associated with an excellent prognosis, the prognosis for patients with residual disease is variable. The mitotic count (MC) is commonly used in the evaluation of histologic tumor grade, but its prognostic value relative to other factors when determined after NST has not been studied. We evaluated MC in the residual tumor after NST in order to determine whether it provided prognostic information independent of other factors, including the residual cancer burden (RCB). We retrospectively reviewed pathologic specimens from 80 patients with localized breast cancer who received standard NST, of whom 61 had residual disease evaluable for MC analysis and RCB score. The exact number of mitotic figures was counted in 10 high power (40×) fields (hpf). We classified tumors as having high (≥13 per 10 hpf) and low (<13 per 10 hpf) MC because this threshold fell at the midpoint for an intermediate MC score in the Nottingham combined histologic grade. Distant metastases developed in 2 of 32 (6.3 %) patients with a low MC compared with 18 of 29 (62.1 %) with a high MC (log-rank test, p < 0.001). When adjusted for other covariates, including age, estrogen receptor, HER2/neu expressions, and RCB score, a high MC was associated with a significantly higher risk of developing distant metastases (hazard ratio 11.21, 95 % CI [2.19, 57.37]; p = 0.004). Our findings indicated that evaluation of MC after NST warrants validation and further evaluation as a prognostic marker in breast cancer.



Similar content being viewed by others
References
Kaufman M, Von Minckwitz G, Mamounas EP et al (2012) Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol 19(5):1508–1516
Von Minckwitz G, Untch M, Blohmer J (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804
Prowell TM, Pazdur R (2012) Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med 336:2438–2441
Sahoo S, Lester SC (2009) Pathology of breast carcinomas after neoadjuvant chemotherapy: an overview with recommendations on specimen processing and reporting. Arch Pathol Lab Med 133:633–642
Symmans WF, Peintinger F, Hatzis C et al (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25(28):4414–4422
Edge SB, Byrd DR, Carducci MA, Compton CC (eds) (2009) AJCC cancer staging manual, 7th edn. Springer, New York
Ellis IO, Elston CW (2006) Histologic grade (chapter 19). In: O’Malley FP, Pinder SE (eds) Breast pathology. Elsevier, Philadelphia, pp 225–233
Lester SC, Bose S, Chen YY et al (2009) Protocol for the examination of specimens from patients with invasive carcinoma of the breast. Arch Pathol Lab Med 133:1515–1538
Hammond EH, Hayes DF, Dowsett M et al (2010) American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med 134:907–922
McShane LM, Altman DG, Sauerbrei W et al (2006) Reporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100:229–235
Thomas E, Holmes FA, Smith TL et al (2004) The use of alternate, non-cross-resistant adjuvant chemotherapy on the basis of pathologic response to a neoadjuvant doxorubicin-based regimen in women with operable breast cancer: long-term results from a prospective randomized trial. J Clin Oncol 22:2294–2302
Wirapati P, Sotiriou C, Kunkel S et al (2008) Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res 10(4):R65
Jones RL, Slater J, A’Hern R et al (2009) The prognostic significance of Ki67 before and and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat 116(1):53–68
Nishimura R, Osdako T, Okumura Y et al (2010) Clinical significance of Ki-67 in neoadjuvant chemotherapy for primary breast cancers as a predictor for chemo sensitivity and for prognosis. Breast Cancer 17:269–275
Penault-Llorca F, Abrial A, Raoelfils I et al (2008) Changes and predictive and prognostic value of mitotic index, Ki-67, Cyclin D1 and cyclo-oxygenase-2 in 710 operable breast cancer patients treated with neoadjuvant chemotherapy. Oncologist 13(12):1235–1245
Yerushalmi R, Woods R, Ravdin P, Malcome MH, Gelman K (2010) Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 11:174–183
Dowsett M, Nielsen TO, A’Hern RA et al (2011) Assessment of Ki67 in breast cancer: recommendations from the international Ki67 in breast cancer working group. J Natl Cancer Inst 103(22):1656–1664
Jonat W, Arnold N (2011) Is the Ki-67 labelling index ready for clinical use? Ann Oncol 22(3):500–502
Acknowledgments
This study was supported in part by Grants from the Department of Health and Human Services and the National Institutes of Health to the Albert Einstein College of Medicine (P30 11130).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diaz, J., Stead, L., Shapiro, N. et al. Mitotic counts in breast cancer after neoadjuvant systemic chemotherapy and development of metastatic disease. Breast Cancer Res Treat 138, 91–97 (2013). https://doi.org/10.1007/s10549-013-2411-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2411-7