Abstract
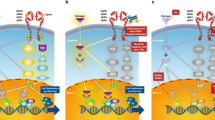
There is considerable pre-clinical and clinical evidence demonstrating that HER2-positive breast cancers that express estrogen receptor (ER) exhibit intrinsic resistance to endocrine therapy. Therefore, in general, chemotherapy in combination with HER2-directed agents is recommended for all but the smallest HER2-positive early stage breast cancers regardless of ER status. This paradigm has recently come into question when responses to neo-adjuvant HER2-directed regimens were noted to vary based on ER expression, and pathologic complete response was noted not to be prognostic for ER-positive, HER2-positive breast cancers. These and other data suggest the possibility that a subset of HER2-positive, ER-positive breast cancers are driven primarily by ER, and biologically behave more like HER2-negative, ER-positive breast cancers. Identification of this subset of HER2-positive breast cancers is essential to avoid over-treatment of patients with small HER2-positive, ER-positive breast cancers, who may be optimally treated with endocrine therapy alone, or in combination with a HER2-directed agent, thereby avoiding the use of chemotherapy. Crosstalk between the ER and HER2 pathways has been established as playing a role in both intrinsic and acquired resistance to endocrine agents. Emerging data suggests that crosstalk between these pathways is also involved in resistance to HER2-directed agents. Unraveling the role of the ER pathway in resistance to HER2-directed agents could potentially result in therapeutic approaches that can improve outcome for patients with ER-positive, HER2-positive breast cancer.




Similar content being viewed by others
References
Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK (1992) Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat 24:85–95
Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, Gorman CM, Parker MG, Sliwkowski MX, Slamon DJ (1995) HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene 10:2435–2446
Chang JCN, Forero-Torres A, Nanda R, Goetz MP, Rodriguez AA, Pavlick AC et al (2011) BCRC 006: a multicenter phase II study of neoadjuvant lapatinib and trastuzumab in patients with HER2-overexpressing breast cancer. J Clin Oncol 29(Suppl)(Abstract 505)
Gianni L, Im Y-H, Roman L, Tseng L-M, Liu M-C, Lluch-Hernandez A et al (2010) Neoadjuvant pertuzumab (P) and trastuzumab (H): antitumor and safety analysis of a randomized phase II study (‘NeoSphere’). Cancer Res 70(24S)(Abstract S3-2)
Guarneri V, Bottini A, Generali DG, Cagossi K, Artioli F, Bisagni G et al (2011) Final results of a phase II randomized trial of neoadjuvant anthracycline-taxane chemotherapy plus lapatinib, trastuzumab, or both in HER2-positive breast cancer (CHER-LOB trial). J Clin Oncol 29(Suppl)(Abstract 507)
Holmes FA, Espina VA, Liotta LA, Chang JC, Nagarwala YM, Danso M et al (2011) Correlation of clinical and molecular findings with pathologic response to preoperative lapatinib and trastuzumab, separately or in combination, prior to neoadjuvant chemotherapy for HER2 positive breast cancer. Cancer Res 71(Abstract P15-3)
Schneeweiss A, Chia, S., Hickish, T., Harvey, V., Eniu, A., Hegg, R., et al. (2011): Neoadjuvant pertuzumab and trastuzumab concurrent or sequential with an anthracycline-containing or concurrent with an anthracycline free standard regimen: a randomised phase II study (TRYPHAENA). Cancer Res 71(Abstract S5-6)
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gomez H, Dinh P, Fauria K, Van Dooren V et al (2012) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379:633–640
Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JU, Hilfrich J, Strumberg D, Fasching PA, Kreienberg R et al (2012) Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol 13:135–144
Bhargava R, Dabbs DJ, Beriwal S, Yildiz IA, Badve P, Soran A, Johnson RR, Brufsky AM, Lembersky BC, McGuire KP et al (2011) Semiquantitative hormone receptor level influences response to trastuzumab-containing neoadjuvant chemotherapy in HER2-positive breast cancer. Mod Pathol 24:367–374
Montemurro F, Rossi V, Rocca MC, Martinello R, Verri E, Redana S, Adamoli L, Valabrega G, Sapino A, Aglietta M et al (2012) Hormone-receptor expression and activity of trastuzumab with chemotherapy in HER2-positive advanced breast cancer patients. Cancer 118:17–26
Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, Arteaga CL (2000) Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res 60:5887–5894
De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, Tortora G, D’Agostino D, Caputo F, Cancello G et al (2005) A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res 11:4741–4748
Johnston S, Pippen J Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ et al (2009) Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 27:5538–5546
Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova A et al (2009) Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 27:5529–5537
Bonneterre J, Buzdar A, Nabholtz JM, Robertson JF, Thurlimann B, von Euler M, Sahmoud T, Webster A, Steinberg M (2001) Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer 92:2247–2258
Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Jaenicke F et al (2003) Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 21:2101–2109
Johnston SR (2005) Combinations of endocrine and biological agents: present status of therapeutic and presurgical investigations. Clin Cancer Res 11:889s–899s
Gluck S, Arteaga CL, Osborne CK (2011) Optimizing chemotherapy-free survival for the ER/HER2-positive metastatic breast cancer patient. Clin Cancer Res 17:5559–5561
Schafer JM, Lee ES, O’Regan RM, Yao K, Jordan VC (2000) Rapid development of tamoxifen-stimulated mutant p53 breast tumors (T47D) in athymic mice. Clin Cancer Res 6:4373–4380
Leary AF, Drury S, Detre S, Pancholi S, Lykkesfeldt AE, Martin LA, Dowsett M, Johnston SR (2010) Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res 16:1486–1497
Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, Weiss H, Rimawi M, Schiff R (2008) Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res 68:826–833
Sabnis G, Schayowitz A, Goloubeva O, Macedo L, Brodie A (2009) Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res 69:1416–1428
Long BJ, Jelovac D, Thiantanawat A, Brodie AM (2002) The effect of second-line antiestrogen therapy on breast tumor growth after first-line treatment with the aromatase inhibitor letrozole: long-term studies using the intratumoral aromatase postmenopausal breast cancer model. Clin Cancer Res 8:2378–2388
Kaklamani VG, Cianfrocca M, Ciccone J, Kindy K, Rademaker A, Wiley EL, Gradishar W, O’Regan RM (2010) Increased HER2/neu expression in recurrent hormone receptor-positive breast cancer. Biomarkers 15:191–193
O’Regan RM, Osipo C, Ariazi E, Lee ES, Meeke K, Morris C, Bertucci A, Sarker MA, Grigg R, Jordan VC (2006) Development and therapeutic options for the treatment of raloxifene-stimulated breast cancer in athymic mice. Clin Cancer Res 12:2255–2263
Baselga J, Campone M, Piccart M, Burris HA, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F et al (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520–529
Bachelot T, Bourgier C, Cropet C, Guastalla J-P, Ferrero J-M, Leger-Falandry C et al (2010) TAMRAD: a GINECO randomized phase II trial of everolimus in combination with tamoxifen versus tamoxifen alone in patients with hormone receptor-positive, HER2-negative metastatic breast cancer with prior exposure to aromatase inhibitors. SABCS:Abstract S1-6
Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch C et al (2011) Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol 12:236–244
Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE Jr, Martino S, Mamounas EP, Kaufman PA, Wolmark N (2011) Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 29:3366–3373
Peron J, Tredan O, Ray-Coquard I, Bachelot T, Labidi Galy SI, Favier B, Treilleux I, Guastalla JP (2012) First-line endocrine therapy alone could be a reasonable treatment option for hormone-positive, HER2-positive metastatic breast cancer. Bull Cancer 99:18–25
Mittendorf EA, Wu Y, Scaltriti M, Meric-Bernstam F, Hunt KK, Dawood S, Esteva FJ, Buzdar AU, Chen H, Eksambi S et al (2009) Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res 15:7381–7388
Esserman LJ, Berry DA, Cheang MC, Yau C, Perou CM, Carey L, Demichele A, Gray JW, Conway-Dorsey K, Lenburg ME et al (2011) Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat. doi:10.1007/s10549-011-1895-2
Untch MFP, Konecny GE, Hasmuller S, Lebeau A, Kreienberg R, Camara O, Muller V, du Bois A, Kuhn T et al (2011) Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol 29:3361–3367
von Minckwitz G, Blohmer JU, Costa S, Denkert C, Eidtmann H, Eiermann W, Gerber B, Hanusch C, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Kümmel S, Paepke S, Schneeweiss A, Untch M, Zahm DM, Mehta K, Loibl S (2011) Neoadjuvant chemotherapy adapted by interim response improves overall survival of primary breast cancer patients—results of the GeparTrio Trial. Cancer Res 71(Abstract S3-2)
Ahn ER, Vogel CL (2012) Dual HER2-targeted approaches in HER2-positive breast cancer. Breast Cancer Res Treat 131:371–383
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B (2001) Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Mongraphs 30:96–102
Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, Brown AM, Robidoux A, Margolese R, Kahlenberg MS et al (2006) Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 24:2019–2027
Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM (2007) The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 13:2329–2334
Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26:1275–1281
Untch M, Fasching PA, Konecny GE, Hasmuller S, Lebeau A, Kreienberg R, Camara O, Muller V, du Bois A, Kuhn T et al (2011) Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol 29:3351–3357
Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, Wilson C, Rong HM, Bauerfeind I, Felber M et al (2003) Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst 95:142–153
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2009) Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 20:1319–1329
Elledge RM, Green S, Pugh R, Allred DC, Clark GM, Hill J, Ravdin P, Martino S, Osborne CK (2000) Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int J Cancer 89:111–117
Rimawi MF, Wiechmann LS, Wang YC, Huang C, Migliaccio I, Wu MF, Gutierrez C, Hilsenbeck SG, Arpino G, Massarweh S et al (2011) Reduced dose and intermittent treatment with lapatinib and trastuzumab for potent blockade of the HER pathway in HER2/neu-overexpressing breast tumor xenografts. Clin Cancer Res 17:1351–1361
Knauer M, Cardoso F, Wesseling J, Bedard PL, Linn SC, Rutgers EJ, van’t Veer LJ (2010) Identification of a low-risk subgroup of HER-2-positive breast cancer by the 70-gene prognosis signature. Br J Cancer 103:1788–1793
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826
Sotiriou C, Pusztai L (2009) Gene-expression signatures in breast cancer. N Engl J Med 360:790–800
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M et al (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Cancer 365:1273–1283
Loibl S, von Minckwitz G, Blohmer JU, Costa SD, Eidtmann H, Fasching PA et al (2011) pCR as a surrogate in HER2-positive patients treated with trastuzumab. Cancer Res 71(Abstract S5-4)
Pinzone JJ, Stevenson H, Strobl JS, Berg PE (2004) Molecular and cellular determinants of estrogen receptor alpha expression. Mol Cell Biol 24:4605–4612
Stoica A, Saceda M, Doraiswamy VL, Coleman C, Martin MB (2000) Regulation of estrogen receptor-alpha gene expression by epidermal growth factor. J Endocrinol 165:371–378
Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, Paulazzo G, Lyass L, Trusk P, Hill J et al (2006) A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci USA 103:7795–7800
Parra-Palau JL, Pedersen K, Peg V, Scaltriti M, Angelini PD, Escorihuela M, Mancilla S, Sanchez Pla A, Ramon YCS, Baselga J et al (2010) A major role of p95/611-CTF, a carboxy-terminal fragment of HER2, in the down-modulation of the estrogen receptor in HER2-positive breast cancers. Cancer Res 70:8537–8546
Garrett JT, Arteaga CL (2011) Resistance to HER2-directed antibodies and tyrosine kinase inhibitors: mechanisms and clinical implications. Cancer Biol Ther 11:793–800
Nahta R, Esteva FJ (2006) HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res 8:215
Valabrega G, Montemurro F, Sarotto I, Petrelli A, Rubini P, Tacchetti C, Aglietta M, Comoglio PM, Giordano S (2005) TGFalpha expression impairs trastuzumab-induced HER2 downregulation. Oncogene 24:3002–3010
du Manoir JM, Francia G, Man S, Mossoba M, Medin JA, Viloria-Petit A, Hicklin DJ, Emmenegger U, Kerbel RS (2006) Strategies for delaying or treating in vivo acquired resistance to trastuzumab in human breast cancer xenografts. Clin Cancer Res 12:904–916
Wang YC, Morrison G, Gillihan R, Guo J, Ward RM, Fu X, Botero MF, Healy NA, Hilsenbeck SG, Phillips GL et al (2011) Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers—role of estrogen receptor and HER2 reactivation. Breast Cancer Res 13:R121
Oh AS, Lorant LA, Holloway JN, Miller DL, Kern FG, El-Ashry D (2001) Hyperactivation of MAPK induces loss of ERalpha expression in breast cancer cells. Mol Endocrinol 15:1344–1359
Holloway JN, Murthy S, El-Ashry D (2004) A cytoplasmic substrate of mitogen-activated protein kinase is responsible for estrogen receptor-alpha down-regulation in breast cancer cells: the role of nuclear factor-kappaB. Mol Endocrinol 18:1396–1410
Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S, Carroll JS (2008) Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature 456:663–666
Conflict of interest
Dr. Nahta has no conflicts to declare. Dr. O’Regan receives research funding from Genentech, Glaxo-Smith-Kline and Novartis, and has served as an advisor for Genomic Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nahta, R., O’Regan, R.M. Therapeutic implications of estrogen receptor signaling in HER2-positive breast cancers. Breast Cancer Res Treat 135, 39–48 (2012). https://doi.org/10.1007/s10549-012-2067-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2067-8