Abstract
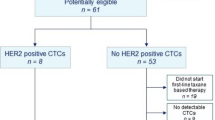
This multicenter phase II trial was designed to evaluate the activity of lapatinib in metastatic breast cancer patients with HER2-negative primary tumors and HER2-positive circulating tumor cells (CTCs). In this study MBC patients with HER2-negative primary tumors and HER2-positive CTCs previously treated with at least a first-line therapy for metastatic disease received lapatinib 1500 mg/day. The CellSearch System® was used for CTCs isolation and bio-characterization. HER2 status was assessed on CTCs by immunofluorescence. A case was defined as CTCs positive if ≥2 CTC/7.5 ml of blood were isolated and HER2-positive if ≥50 % of CTCs were HER2-positive. 139 HER2-negative patients were screened, 96 patients were positive for CTCs (mean number of CTCs: 85; median number of CTCs: 19; range 2–1637). Seven of the 96 patients (7 %) had ≥50 % HER2-positive CTCs and were eligible for treatment with lapatinib. No objective tumor responses occurred in this population. In one patient, disease stabilization lasting 254 days (8.5 months) was observed. From the findings of this study, we concluded that a subset of patients with a HER2-negative primary tumor presents HER2-positive CTCs during disease progression, although the HER2 shift rate seems to be lower than previously reported. Despite the lack of objective response, the durable disease stabilization observed in one patient cannot rule out the hypothesis that lapatinib may have some activity in this patient population. However, considering that only 1/139 screened patients may potentially have derived benefit from this approach, future trials designed according to the presented strategy cannot be recommended.


Similar content being viewed by others
References
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Zidan J, Dashkovsky I, Stayerman C, Basher W, Cozacov C, Hadary A (2005) Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer 93(5):552–556
Edgerton SM, Moore D 2nd, Merkel D, Thor AD (2003) erbB-2 (HER-2) and breast cancer progression. Appl Immunohistochem Mol Morphol 11(3):214–221
Gancberg D, Di Leo A, Cardoso F, Rouas G, Pedrocchi M, Paesmans M et al (2002) Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann Oncol 13(7):1036–1043
Curigliano G, Bagnardi V, Viale G, Fumagalli L, Rotmensz N, Aurilio G et al (2011) Should liver metastases of breast cancer be biopsied to improve treatment choice? Ann Oncol 22(10):2227–2233
Amir E, Miller N, Geddie W, Freedman O, Kassam F, Simmons C et al (2012) Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol 30(6):587–592
Amir E, Clemons M, Purdie CA, Miller N, Quinlan P, Geddie W et al (2011) Tissue confirmation of disease recurrence in breast cancer patients: pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treat Rev
Fehm T, Sagalowsky A, Clifford E, Beitsch P, Saboorian H, Euhus D et al (2002) Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin Cancer Res 8(7):2073–2084
Terstappen LW, Rao C, Gross S, Weiss AJ (2000) Peripheral blood tumor cell load reflects the clinical activity of the disease in patients with carcinoma of the breast. Int J Oncol 17(3):573–578
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC et al (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8):781–789
Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM et al (2005) Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 23(7):1420–1430
Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Perkins S et al (2004) HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci U S A 101(25):9393–9398
Riethdorf S, Müller V, Zhang L, Rau T, Loibl S, Komor M et al (2010) Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res 16(9):2634–2645
Fehm T, Müller V, Aktas B, Janni W, Schneeweiss A, Stickeler E et al (2010) HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat 124(2):403–412
Munzone E, Nolé F, Goldhirsch A, Botteri E, Esposito A, Zorzino L et al (2010) Changes of HER2 status in circulating tumor cells compared with the primary tumor during treatment for advanced breast cancer. Clin Breast Cancer 10(5):392–397
Flores LM, Kindelberger DW, Ligon AH, Capelletti M, Fiorentino M, Loda M et al (2010) Improving the yield of circulating tumour cells facilitates molecular characterisation and recognition of discordant HER2 amplification in breast cancer. Br J Cancer 102(10):1495–1502
Punnoose EA, Atwal SK, Spoerke JM, Savage H, Pandita A, Yeh RF et al (2010) Molecular biomarker analyses using circulating tumor cells. PLoS ONE 5(9):e12517
Pestrin M, Bessi S, Galardi F, Truglia M, Biggeri A, Biagioni C et al (2009) Correlation of HER2 status between primari tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res Treat 118(3):523–530
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Sargent DJ, Chan V, Goldberg RM (2001) A three-outcome design for phase II clinical trials. Control Clin Trials 22:117–125
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C et al (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10(20):6897–6904
Riethdorf S, Fritsche H, Müller V, Rau T, Schindlbeck C, Rack B et al (2007) Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 13(3):920–928
Press MF, Slamon DJ, Flom KJ, Park J, Zhou J-Y, Bernstein L (2002) Evaluation of HER-2/neu gene amplification and overexpression: comparison of frequently used assay methods in a molecularly characterized cohort of breast cancer specimens. J Clin Oncol 20:3095–3105
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ et al (2007) American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145
Guarneri V, Giovannelli S, Ficarra G, Bettelli S, Maiorana A, Piacentini F et al (2008) Comparison of HER-2 and hormone receptor expression in primary breast cancers and asynchronous paired metastases: impact on patient management. Oncologist 13(8):838–844
Meng S, Tripathy D, Shete S, Ashfaq R, Saboorian H, Haley B et al (2006) uPAR and HER-2 gene status in individual breast cancer cells from blood and tissues. Proc Natl Acad Sci U S A 103(46):17361–17365
Solomayer EF, Becker S, Pergola-Becker G, Bachmann R, Krämer B, Vogel U et al (2006) Comparison of HER2 status between primary tumor and disseminated tumor cells in primary breast cancer patients. Breast Cancer Res Treat 98(2):179–184
Conflict of interest
Angelo Di Leo (Author # 16) and Fabio Puglisi (Author # 3) declare a consultant/advisory role with GlaxoSmithKline® and Roche®. Lorenzo Gianni (Author # 8) declares a consultant/advisory role with GlaxoSmithKline®. Other Authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical trials.gov identifier: NCT00820924.
Rights and permissions
About this article
Cite this article
Pestrin, M., Bessi, S., Puglisi, F. et al. Final results of a multicenter phase II clinical trial evaluating the activity of single-agent lapatinib in patients with HER2-negative metastatic breast cancer and HER2-positive circulating tumor cells. A proof-of-concept study. Breast Cancer Res Treat 134, 283–289 (2012). https://doi.org/10.1007/s10549-012-2045-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2045-1