Abstract
Forkhead box protein A1 (FOXA1) is a “pioneer factor” that plays a role in controlling nearly 50% of estrogen receptor target genes. FOXA1 expression correlates with estrogen receptor (ER)-positivity especially in luminal subtype A breast cancers. The aim of this study was to investigate the precise role of FOXA1 in breast cancer using a large population-based cohort. Nuclear expression of FOXA1 was analyzed in a tissue microarray of 4,444 invasive breast cancer cases using immunohistochemistry and correlated with clinicopathologic variables using previously described methods and cutoff points. The entire cohort was equally divided into a training and validation set. All survival analyses were performed using a previously defined cutoff (3) for validation. Additional X-tile analysis performed to analyze prognostic effects of low and high FOXA1 levels identified 24 as a cutoff. Bonferroni–Holmes test was used as appropriate. FOXA1 expression significantly correlated positively with markers of good prognosis or ER-positivity, and negatively with tumor size, tumor grade, nodal status, Ki67, HER2 expression, and basal subtype (each P value <0.0001). In both survival analyses, FOXA1 was a significant predictor of breast cancer-specific survival (P < 0.0001) and relapse-free survival (P < 0.0001). FOXA1 was also an independent predictor of breast cancer-specific survival at 10 years using both cutoffs. Among the ER-positive subgroup treated with tamoxifen, FOXA1 was an independent prognostic marker using the 24 cutoff (P = 0.030). FOXA1 is a significant marker of good prognosis in breast cancer; it also identifies a subset of ER-positive tamoxifen treated patients at low risk of recurrence.



Similar content being viewed by others
References
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752
Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M (2005) Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FOXA1. Cell 122(1):33–43
Laganiere J, Deblois G, Lefebvre C, Bataille AR, Robert F, Giguere V (2005) From the cover: location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc Natl Acad Sci 102(33):11651–11656
Costa RH, Grayson DR, Darnell JE Jr (1989) Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol 9(4):1415–1425
Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS (2011) FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet 43(1):27–33
Clark KL, Halay ED, Lai E, Burley SK (1993) Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature 364(6436):412–420
Carroll JS, Brown M (2006) Estrogen receptor target gene: an evolving concept. Mol Endocrinol 20(8):1707–1714
Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z (2006) GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 127(5):1041–1055
Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, Lindeman GJ, Visvader JE (2007) GATA-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 9(2):201–209
Ademuyiwa FO, Thorat MA, Jain RK, Nakshatri H, Badve S (2010) Expression of Forkhead-box protein A1, a marker of luminal A type breast cancer, parallels low Oncotype DX 21-gene recurrence scores. Mod Pathol 23(2):270–275
Wolf I, Bose S, Williamson EA, Miller CW, Karlan BY, Koeffler HP (2007) FOXA1: growth inhibitor and a favorable prognostic factor in human breast cancer. Int J Cancer 120(5):1013–1022
Williamson EA, Wolf I, O’Kelly J, Bose S, Tanosaki S, Koeffler HP (2006) BRCA1 and FOXA1 proteins coregulate the expression of the cell cycle-dependent kinase inhibitor p27(Kip1). Oncogene 25(9):1391–1399
West M, Blanchette C, Dressman H, Huang E, Ishida S, Spang R, Zuzan H, Olson JA Jr, Marks JR, Nevins JR (2001) Predicting the clinical status of human breast cancer by using gene expression profiles. Proc Natl Acad Sci 98(20):11462–11467
Thorat MA, Marchio C, Morimiya A, Savage K, Nakshatri H, Reis-Filho JS, Badve S (2008) Forkhead box A1 expression in breast cancer is associated with luminal subtype and good prognosis. J Clin Pathol 61(3):327–332
Badve S, Turbin D, Thorat MA, Morimiya A, Nielsen TO, Perou CM, Dunn S, Huntsman DG, Nakshatri H (2007) FOXA1 expression in breast cancer—correlation with luminal subtype A and survival. Clin Cancer Res 13(15 Pt 1):4415–4421
Nakshatri H, Badve S (2007) FOXA1 as a therapeutic target for breast cancer. Expert Opin Ther Targets 11(4):507–514
Albergaria A, Paredes J, Sousa B, Milanezi F, Carneiro V, Bastos J, Costa S, Vieira D, Lopes N, Lam EW, Lunet N, Schmitt F (2009) Expression of FOXA1 and GATA-3 in breast cancer: the prognostic significance in hormone receptor-negative tumours. Breast Cancer Res 11(3):R40
Habashy HO, Powe DG, Rakha EA, Ball G, Paish C, Gee J, Nicholson RI, Ellis IO (2008) Forkhead-box A1 (FOXA1) expression in breast cancer and its prognostic significance. Eur J Cancer 44(11):1541–1551
Chia SK, Speers CH, Bryce CJ, Hayes MM, Olivotto IA (2004) Ten-year outcomes in a population-based cohort of node-negative, lymphatic, and vascular invasion-negative early breast cancers without adjuvant systemic therapies. J Clin Oncol 22(9):1630–1637
Lohrisch C, Jackson J, Jones A, Mates D, Olivotto IA (2000) Relationship between tumor location and relapse in 6,781 women with early invasive breast cancer. J Clin Oncol 18(15):2828–2835
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4(7):844–847
Parker RL, Huntsman DG, Lesack DW, Cupples JB, Grant DR, Akbari M, Gilks CB (2002) Assessment of interlaboratory variation in the immunohistochemical determination of estrogen receptor status using a breast cancer tissue microarray. Am J Clin Pathol 117(5):723–728
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101(10):736–750
Cheang MC, Treaba DO, Speers CH, Olivotto IA, Bajdik CD, Chia SK, Goldstein LC, Gelmon KA, Huntsman D, Gilks CB, Nielsen TO, Gown AM (2006) Immunohistochemical detection using the new rabbit monoclonal antibody SP1 of estrogen receptor in breast cancer is superior to mouse monoclonal antibody 1D5 in predicting survival. J Clin Oncol 24(36):5637–5644
Chia S, Norris B, Speers C, Cheang M, Gilks B, Gown AM, Huntsman D, Olivotto IA, Nielsen TO, Gelmon K (2008) Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol 26(35):5697–5704
Crabb SJ, Bajdik CD, Leung S, Speers CH, Kennecke H, Huntsman DG, Gelmon KA (2008) Can clinically relevant prognostic subsets of breast cancer patients with four or more involved axillary lymph nodes be identified through immunohistochemical biomarkers? A tissue microarray feasibility study. Breast Cancer Res 10(1):R6
Voduc D, Cheang M, Nielsen T (2008) GATA-3 expression in breast cancer has a strong association with estrogen receptor but lacks independent prognostic value. Cancer Epidemiol Biomarkers Prev 17(2):365–373
Wong MP, Cheang M, Yorida E, Coldman A, Gilks CB, Huntsman D, Berean K (2008) Loss of desmoglein 1 expression associated with worse prognosis in head and neck squamous cell carcinoma patients. Pathology 40(6):611–616
Eeckhoute J, Lupien M, Meyer CA, Verzi MP, Shivdasani RA, Liu XS, Brown M (2009) Cell-type selective chromatin remodeling defines the active subset of FOXA1-bound enhancers. Genome Res 19(3):372–380
Badve S, Nakshatri H (2009) Oestrogen-receptor-positive breast cancer: towards bridging histopathological and molecular classifications. J Clin Pathol 62(1):6–12
Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, Gerald WL (2006) An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 25(28):3994–4008
Yamaguchi N, Ito E, Azuma S, Honma R, Yanagisawa Y, Nishikawa A, Kawamura M, Imai J, Tatsuta K, Inoue J, Semba K, Watanabe S (2008) FOXA1 as a lineage-specific oncogene in luminal type breast cancer. Biochem Biophys Res Commun 365(4):711–717
Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H (2007) NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene 26(5):711–724
Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, Fehrenbacher L, Lin Gomez S, Miles S, Neugut AI (2010) Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 28(27):4120–4128
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25(33):5287–5312
Acknowledgments
The Genetic Pathology Evaluation Centre is supported by an unrestricted educational grant from Sanofi-aventis Canada. We thank Caroline Speers and members of the Breast Cancer Outcomes Unit for clinical database management. The authors would also like to thank Dr. Kathy Miller, Indiana University for critical review and comments.
Conflicts of interest
Authors HN and SB have an invention disclosure with Indiana University for the use of FOXA1 as a prognostic marker in breast cancer. Both SB and HN are supported by grants from Susan G Komen Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Description of the cohort of 4046 breast cancer patients for important clinic-pathological variables. (DOCX 14 kb)
Supplementary Table 2
Description of univariate survival analyses for important variables in the entire cohort (n = 4046). (DOC 47 kb)
Supplementary Table 3
Correlation of FOXA1 categories (<3 vs. ≥3) with important clinic-pathological variables in training and validation cohorts. (DOCX 16 kb)
Supplementary Table 4
Correlation of FOXA1 categories (<3 vs. ≥3) with important clinic-pathological variables in the entire cohort. (DOCX 15 kb)
Supplementary Table 5
Description of survival analyses comparing low versus high FOXA1 expression in 4,046 breast cancer cases with longer follow-up. This table has two parts; top portion displaying results of univariate analyses and the bottom portion displaying the various models used for multivariate analysis. All results have been shown for both Parts A and B of the survival analyses with a longer followup. (DOCX 16 kb)
Supplementary Table 6
Table showing P-values after Bonferroni–Holmes correction for associations of FOXA1 expression with different clinicopathologic variables in training, validation, and entire cohort. *All survival analyses have been shown primarily for 10 years time period unless stated otherwise. (XLSX 12 kb)
Supplementary Figure 1
Schematic representation of the study design; (A) shows how the 3 cutoff was used in this study that was initially derived from our previous work and (B) shows how the 24 cutoff was derived from a separate X-tile analysis of this cohort. (PPTX 39 kb)
Supplementary Figure 2
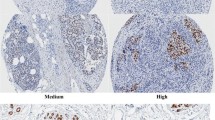
Representative staining of FOXA1 in breast cancer using immunohistochemistry; A- no expression vs. B- strong expression (PPTX 726 kb)
Supplementary Figure 3
Kaplan–Meier curve demonstrating impact of FOXA1 expression on BCSS and RFS at 20 years. The top panel displays the survival functions using a 3 cutoff while the same with a 24 cutoff has been shown in the bottom panel. The curves clearly show that cases with high FOXA1 expression (either >3 or >24) have a better prognosis than those with low expression. This identifies the prognostic impact of FOXA1 in breast cancer. (PPTX 272 kb)
Supplementary Figure 4
Kaplan–Meier curves demonstrating impact of FOXA1 expression on survival among ER-positive cases not receiving any systemic therapy. This identifies the ability of FOXA1 to be an important marker among ER-positive cases that have good prognosis even in the absence of any systemic therapy. High FOXA1 expression (either >3 or >24) has better prognosis than low expression in this subgroup. (PPTX 129 kb)
Supplementary Figure 5
Kaplan–Meier curves demonstrating impact of FOXA1 in all ER-positive patients. These curves show that high FOXA1 expression (either >3 or >24) have better prognosis than those with low expression in ER-positive patients. No treatment subgroups have been considered for this particular analysis. (PPTX 142 kb)
Rights and permissions
About this article
Cite this article
Mehta, R.J., Jain, R.K., Leung, S. et al. FOXA1 is an independent prognostic marker for ER-positive breast cancer. Breast Cancer Res Treat 131, 881–890 (2012). https://doi.org/10.1007/s10549-011-1482-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1482-6