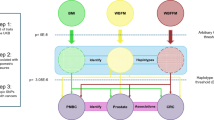
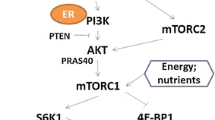
Abstract
AMP-activated protein kinase (AMPK) is an energy sensing/signalling intracellular protein which is activated by an increase in the cellular AMP:ATP ratio after ATP depletion. Once activated, AMPK inhibits fatty acid synthesis and the Akt-mTOR pathway, and activates the p53-p21 axis. All these molecular mechanisms are thought to play a key role in breast carcinogenesis. We investigated the genetic variability of four genes encoding AMPK (PRKAA1, PRKAA2, PRKAB1 and PRKAB2). Using a tagging approach and selecting SNPs we covered all the common genetic variation of these genes. We tested association of tagging SNPs in our four candidate genes with breast cancer (BC) risk in a study of 1340 BC cases and 2536 controls nested into the European Prospective Investigation into Cancer and Nutrition (EPIC). Given the relevance of AMPK on fatty acid synthesis and the importance of body fatness as a BC risk factor, we tested association of SNPs and body-mass index as well. We observed no statistically significant association between the SNPs in the PRKAs genes and BC risk and BMI after correction for multiple testing.
Similar content being viewed by others
References
Augustin LS, Dal Maso L, La Vecchia C, Parpinel M, Negri E, Vaccarella S, Kendall CW, Jenkins DJ, Francesch S (2001) Dietary glycemic index and glycemic load, and breast cancer risk: a case–control study. Ann Oncol 12(11):1533–1538
Bianchini F, Kaaks R, Vainio H (2002) Overweight, obesity, and cancer risk. Lancet Oncol 3(9):565–574. doi:S1470204502008495[pii]
Kaaks R (1996) Nutrition, hormones, and breast cancer: is insulin the missing link? Cancer Causes Control 7(6):605–625
Kaaks R, Lukanova A (2001) Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc 60(1):91–106. doi:S002966510100012X[pii]
Muti P, Quattrin T, Grant BJ, Krogh V, Micheli A, Schunemann HJ, Ram M, Freudenheim JL, Sieri S, Trevisan M, Berrino F (2002) Fasting glucose is a risk factor for breast cancer: a prospective study. Cancer Epidemiol Biomarkers Prev 11(11):1361–1368
Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA (2003) AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans 31(Pt 1):162–168
Carling D (2005) AMP-activated protein kinase: balancing the scales. Biochimie 87(1):87–91
Hardie DG (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8(10):774–785
Hardie DG, Scott JW, Pan DA, Hudson ER (2003) Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546(1):113–120
Towler MC, Hardie DG (2007) AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100(3):328–341
Hardie DG (1992) Regulation of fatty acid and cholesterol metabolism by the AMP-activated protein kinase. Biochim Biophys Acta 1123(3):231–238
Campa D, McKay J, Sinilnikova O, Husing A, Vogel U, Hansen RD, Overvad K, Witt PM, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Rohrmann S, Chang-Claude J, Boeing H, Fisher E, Trichopoulou A, Trichopoulos D, Palli D, Villarini A, Sacerdote C, Mattiello A, Tumino R, Peeters PH, van Gils CH, Bas Bueno-de-Mesquita H, Lund E, Chirlaque MD, Sala N, Suarez LR, Barricarte A, Dorronsoro M, Sanchez MJ, Lenner P, Hallmans G, Tsilidis K, Bingham S, Khaw KT, Gallo V, Norat T, Riboli E, Rinaldi S, Lenoir G, Tavtigian SV, Canzian F, Kaaks R (2009) Genetic variation in genes of the fatty acid synthesis pathway and breast cancer risk. Breast Cancer Res Treat 118:565–574
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7(10):763–777. doi:nrc2222[pii]10.1038/nrc2222
Menendez JA, Colomer R, Lupu R (2005) Why does tumor-associated fatty acid synthase (oncogenic antigen-519) ignore dietary fatty acids? Med Hypotheses 64(2):342–349. doi:S0306-9877(04)00455-4[pii]10.1016/j.mehy.2004.07.022
Rossi S, Ou W, Tang D, Bhattacharya N, Dei Tos AP, Fletcher JA, Loda M (2006) Gastrointestinal stromal tumours overexpress fatty acid synthase. J Pathol 209(3):369–375
Takahiro T, Shinichi K, Toshimitsu S (2003) Expression of fatty acid synthase as a prognostic indicator in soft tissue sarcomas. Clin Cancer Res 9(6):2204–2212
Yang YA, Morin PJ, Han WF, Chen T, Bornman DM, Gabrielson EW, Pizer ES (2003) Regulation of fatty acid synthase expression in breast cancer by sterol regulatory element binding protein-1c. Exp Cell Res 282(2):132–137. doi:S001448270200023X[pii]
Cleary MP, Grossmann ME (2009) Minireview: obesity and breast cancer: the estrogen connection. Endocrinology 150(6):2537–2542
Cleary MP, Grossmann ME, Ray A (2010) Effect of obesity on breast cancer development. Vet Pathol 47(2):202–213
Gonzalez CA, Riboli E (2010) Diet and cancer prevention: contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer 46(14):2555–2562
Rose DP, Vona-Davis L (2010) Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas 66(1):33–38
Sieri S, Krogh V, Ferrari P, Berrino F, Pala V, Thiebaut AC, Tjonneland A, Olsen A, Overvad K, Jakobsen MU, Clavel-Chapelon F, Chajes V, Boutron-Ruault MC, Kaaks R, Linseisen J, Boeing H, Nothlings U, Trichopoulou A, Naska A, Lagiou P, Panico S, Palli D, Vineis P, Tumino R, Lund E, Kumle M, Skeie G, Gonzalez CA, Ardanaz E, Amiano P, Tormo MJ, Martinez-Garcia C, Quiros JR, Berglund G, Gullberg B, Hallmans G, Lenner P, Bueno-de-Mesquita HB, van Duijnhoven FJ, Peeters PH, van Gils CH, Key TJ, Crowe FL, Bingham S, Khaw KT, Rinaldi S, Slimani N, Jenab M, Norat T, Riboli E (2008) Dietary fat and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 88(5):1304–1312
Hudes GR (2007) mTOR as a target for therapy of renal cancer. Clin Adv Hematol Oncol 5(10):772–774
Rubio-Viqueira B, Hidalgo M (2006) Targeting mTOR for cancer treatment. Curr Opin Investig Drugs 7(6):501–512
Motoshima H, Goldstein BJ, Igata M, Araki E (2006) AMPK and cell proliferation–AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol 574(Pt 1):63–71
Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, Matsumoto K, Toyonaga T, Asano T, Nishikawa T, Araki E (2005) Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res 97(8):837–844
Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB (2005) AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 18(3):283–293
Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H (2001) Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun 287(2):562–567
Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, Overvad K, Tjonneland A, Clavel-Chapelon F, Thiebaut A, Wahrendorf J, Boeing H, Trichopoulos D, Trichopoulou A, Vineis P, Palli D, Bueno-De-Mesquita HB, Peeters PH, Lund E, Engeset D, Gonzalez CA, Barricarte A, Berglund G, Hallmans G, Day NE, Key TJ, Kaaks R, Saracci R (2002) European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr 5(6B):1113–1124. doi:10.1079/PHN2002394S1368980002001350[pii]
Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, Nickerson DA (2004) Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet 74(1):106–120
de Bakker PI, Yelensky R, Pe’er I, Gabriel SB, Daly MJ, Altshuler D (2005) Efficiency and power in genetic association studies. Nat Genet 37(11):1217–1223. doi:ng1669[pii]10.1038/ng1669
Jurinke C, van den Boom D, Cantor CR, Koster H (2001) Automated genotyping using the DNA MassArray technology. Methods Mol Biol 170:103–116 (Clifton NJ)
Little J, Higgins JP, Ioannidis JP, Moher D, Gagnon F, von Elm E, Khoury MJ, Cohen B, Davey-Smith G, Grimshaw J, Scheet P, Gwinn M, Williamson RE, Zou GY, Hutchings K, Johnson CY, Tait V, Wiens M, Golding J, van Duijn C, McLaughlin J, Paterson A, Wells G, Fortier I, Freedman M, Zecevic M, King R, Infante-Rivard C, Stewart A, Birkett N (2009) STrengthening the REporting of Genetic Association studies (STREGA)–an extension of the STROBE statement. Eur J Clin Invest 39(4):247–266
Hardie DG (2005) New roles for the LKB1– >AMPK pathway. Curr Opin Cell Biol 17(2):167–173
Luo Z, Saha AK, Xiang X, Ruderman NB (2005) AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci 26(2):69–76
Brown KA, Hunger NI, Docanto M, Simpson ER (2010) Metformin inhibits aromatase expression in human breast adipose stromal cells via stimulation of AMP-activated protein kinase. Breast Cancer Res Treat 123(2):591–596
Gonzalez-Angulo AM, Meric-Bernstam F (2010) Metformin: a therapeutic opportunity in breast cancer. Clin Cancer Res 16(6):1695–1700
Fortunati N, Catalano MG, Boccuzzi G, Frairia R (2010) Sex Hormone-Binding Globulin (SHBG), estradiol and breast cancer. Mol Cell Endocrinol 316(1):86–92
Jerry DJ, Dunphy KA, Hagen MJ (2010) Estrogens, regulation of p53 and breast cancer risk: a balancing act. Cell Mol Life Sci 67:1017–1023
Mantovani A, Marchesi F, Porta C, Sica A, Allavena P (2007) Inflammation and cancer: breast cancer as a prototype. Breast (Edinburgh, Scotland) 16 Suppl 2:S27–S33
Acknowledgments
Specific results of this study were obtained with financial support from the US Army Medical Research and Material Command (W81XWH-04-1-0271). The EPIC study was funded by “Europe Against Cancer” Programme of the European Commission (SANCO); Ligue contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM); German Cancer Aid; German Cancer Research Center; German Federal Ministry of Education and Research; Danish Cancer Society; Health Research Fund (FIS) of the Spanish Ministry of Health; the participating regional governments and institutions of Spain; Cancer Research UK; Medical Research Council, UK; Hellenic Ministry of Health and Social Solidarity; the Stavros Niarchos Foundation and the Hellenic Health Foundation; Italian Association for Research on Cancer; Italian National Research Council; Dutch Ministry of Public Health, Welfare and Sports (VWS), Dutch Ministry of Health, Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF) (The Netherlands); Statistics Netherlands; Swedish Cancer Society; Swedish Scientific Council; Regional Government of Skane, Sweden; Norwegian Cancer Society.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2010_1269_MOESM1_ESM.xls
Supplementary Table 1. Main effects of 39 SNPs genotyped in the study on breast cancer risk. Columns in this table show: gene name; NCBI dbSNP rs number; the three possible genotypes; numbers of cases for each of the three genotypes; controls for each of the three genotypes; odds ratios for heterozygotes and odds ratios for homozygotes for the rare allele with 95% confidence interval (referred to the homozygotes for the common allele); associated P value; P value of the trend test. (XLS 34 kb)
10549_2010_1269_MOESM2_ESM.xls
Supplementary Table 2. Main effects of 39 SNPs genotyped in the study on BMI. Columns in this table show: gene name; NCBI dbSNP rs number; possible genotypes; numbers of subjects for each of the three genotypes; BMI mean for each of the three genotypes with 95% confidence interval; P value of the trend test. (XLS 31 kb)
Rights and permissions
About this article
Cite this article
Campa, D., Claus, R., Dostal, L. et al. Variation in genes coding for AMP-activated protein kinase (AMPK) and breast cancer risk in the European Prospective Investigation on Cancer (EPIC). Breast Cancer Res Treat 127, 761–767 (2011). https://doi.org/10.1007/s10549-010-1269-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1269-1