Abstract
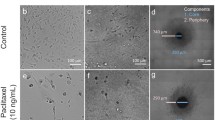
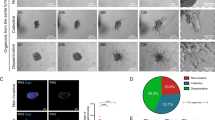
Transforming growth factor-β (TGF-β) has opposing roles in breast cancer progression by acting as a tumor suppressor in the initial phase, but stimulating invasion and metastasis at later stages. In contrast to the mechanisms by which TGF-β induces growth arrest, the pathways that mediate tumor invasion are not well understood. Here, we describe a TGF-β-dependent invasion assay system consisting of spheroids of MCF10A1 normal breast epithelial cells (M1) and RAS-transformed (pre-)malignant derivatives (M2 and M4) embedded in collagen gels. Both basal and TGF-β-induced invasion of these cell lines was found to correlate with their tumorigenic potential; M4 showing the most aggressive behavior and M1 showing the least. Basal invasion was strongly inhibited by the TGF-β receptor kinase inhibitor SB-431542, indicating the involvement of autocrine TGF-β or TGF-β-like activity. TGF-β-induced invasion in premalignant M2 and highly malignant M4 cells was also inhibited upon specific knockdown of Smad3 or Smad4. Interestingly, both a broad spectrum matrix metalloproteinase (MMP) inhibitor and a selective MMP2 and MMP9 inhibitor mitigated TGF-β-induced invasion of M4 cells, while leaving basal invasion intact. In line with this, TGF-β was found to strongly induce MMP2 and MMP9 expression in a Smad3- and Smad4-dependent manner. This collagen-embedded spheroid system therefore offers a valuable screening model for TGF-β/Smad- and MMP2- and MMP9-dependent breast cancer invasion.




Similar content being viewed by others
References
Massagué J (2008) TGFβ in cancer. Cell 134:215–230. doi:10.1016/j.cell.2008.07.001
Akhurst RJ, Derynck R (2001) TGF-β signaling in cancer—a double-edged sword. Trends Cell Biol 11:S44–S51. doi:10.1016/S0962-8924(01)02130-4
Ghellal A, Li C, Hayes M, Byrne G, Bundred N, Kumar S (2000) Prognostic significance of TGFβ1 and TGFβ3 in human breast carcinoma. Anticancer Res 20:4413–4418
Sheen-Chen SM, Chen HS, Sheen CW, Eng HL, Chen WJ (2001) Serum levels of transforming growth factor β1 in patients with breast cancer. Arch Surg 136:937–940
Ivanovic V, Todorovic-Rakovic N, Demajo M, Neskovic-Konstantinovic Z, Subota V, Ivanisevic-Milovanovic O, Nikolic-Vukosavljevic D (2003) Elevated plasma levels of transforming growth factor-β1 (TGF-β1) in patients with advanced breast cancer: association with disease progression. Eur J Cancer 39:454–461. doi:10.1016/S0959-8049(02)00502-6
Desruisseau S, Palmari J, Giusti C, Romain S, Martin PM, Berthois Y (2006) Determination of TGFβ1 protein level in human primary breast cancers and its relationship with survival. Br J Cancer 94:239–246. doi:10.1038/sj.bjc.6602920
ten Dijke P, Hill CS (2004) New insights into TGF-β-Smad signalling. Trends Biochem Sci 29:265–273. doi:10.1016/j.tibs.2004.03.008
Moustakas A, Heldin CH (2009) The regulation of TGFβ signal transduction. Development 136:3699–3714. doi:10.1242/dev.030338
Chen CR, Kang Y, Massagué J (2001) Defective repression of c-myc in breast cancer cells: a loss at the core of the transforming growth factor β growth arrest program. Proc Natl Acad Sci USA 98:992–999
Hannon GJ, Beach D (1994) p15INK4B is a potential effector of TGF-β-induced cell cycle arrest. Nature 371:257–261. doi:10.1038/371257a0
Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF (1995) Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA 92:5545–5549
Levy L, Hill CS (2006) Alterations in components of the TGF-β superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev 17:41–58. doi:10.1016/j.cytogfr.2005.09.009
Gomis RR, Alarcon C, Nadal C, Van PC, Massagué J (2006) C/EBPβ at the core of the TGFβ cytostatic response and its evasion in metastatic breast cancer cells. Cancer Cell 10:203–214. doi:10.1016/j.ccr.2006.07.019
Wakefield LM, Piek E, Bottinger EP (2001) TGF-beta signaling in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia 6:67–82. doi:10.1023/A:1009568532177
Dumont N, Arteaga CL (2000) Transforming growth factor-β and breast cancer: tumor promoting effects of transforming growth factor-β. Breast Cancer Res 2:125–132. doi:10.1186/bcr44
ten Dijke P, Goumans MJ, Itoh F, Itoh S (2002) Regulation of cell proliferation by Smad proteins. J Cell Physiol 191:1–16. doi:10.1002/jcp.10066
Deckers M, van DM, Buijs J, Que I, Lowik C, van der Pluijm G, ten Dijke P (2006) The tumor suppressor Smad4 is required for transforming growth factor β-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res 66:2202–2209. doi:10.1158/0008-5472.CAN-05-3560
Viloria-Petit AM, David L, Jia JY, Erdemir T, Bane AL, Pinnaduwage D, Roncari L, Narimatsu M, Bose R, Moffat J, Wong JW, Kerbel RS, O’Malley FP, Andrulis IL, Wrana JL (2009) A role for the TGFβ-Par6 polarity pathway in breast cancer progression. Proc Natl Acad Sci USA 106:14028–14033. doi:10.1073/pnas.0906796106
Xu J, Lamouille S, Derynck R (2009) TGF-β-induced epithelial to mesenchymal transition. Cell Res 19:156–172. doi:10.1038/cr.2009.5
Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S (1980) Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 284:67–68. doi:10.1038/284067a0
Weigelt B, Peterse JL, van’t Veer LJ (2005) Breast cancer metastasis: markers and models. Nat Rev Cancer 5:591–602. doi:10.1038/nrc1670
Overall CM, Kleifeld O (2006) Tumour microenvironment—opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer 6:227–239. doi:10.1038/nrc1821
Soule HD, Maloney TM, Wolman SR, Peterson WD Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC (1990) Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res 50:6075–6086
Strickland LB, Dawson PJ, Santner SJ, Miller FR (2000) Progression of premalignant MCF10AT generates heterogeneous malignant variants with characteristic histologic types and immunohistochemical markers. Breast Cancer Res Treat 64:235–240. doi:10.1023/A:1026562720218
Santner SJ, Dawson PJ, Tait L, Soule HD, Eliason J, Mohamed AN, Wolman SR, Heppner GH, Miller FR (2001) Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast Cancer Res Treat 65:101–110. doi:10.1023/A:1006461422273
Kim ES, Kim MS, Moon A (2004) TGF-β-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol 25:1375–1382
Miyazono K (2009) Transforming growth factor-β signaling in epithelial-mesenchymal transition and progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci 85:314–323. doi:10.2183/pjab.85.314
Smalley KS, Lioni M, Herlyn M (2006) Life isn’t flat: taking cancer biology to the next dimension. In Vitro Cell Dev Biol Anim 42:242–247. doi:10.1290/0604027.1
Lin RZ, Chang HY (2008) Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J 3:1172–1184. doi:10.1002/biot.200700228
Ohmori T, Yang JL, Price JO, Arteaga CL (1998) Blockade of tumor cell transforming growth factor-βs enhances cell cycle progression and sensitizes human breast carcinoma cells to cytotoxic chemotherapy. Exp Cell Res 245:350–359. doi:10.1006/excr.1998.4261
Graham CH, Kobayashi H, Stankiewicz KS, Man S, Kapitain SJ, Kerbel RS (1994) Rapid acquisition of multicellular drug resistance after a single exposure of mammary tumor cells to antitumor alkylating agents. J Natl Cancer Inst 86:975–982
Petersen M, Pardali E, van der Horst G, Cheung H, van den Hoogen C, van der Pluijm G, ten Dijke P (2010) Smad2 and Smad3 have opposing roles in breast cancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene 29:1351–1361. doi:10.1038/onc.2009.426
Tang B, Vu M, Booker T, Santner SJ, Miller FR, Anver MR, Wakefield LM (2003) TGF-β switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest 112:1116–1124. doi:10.1172/JCI18899
Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS (2002) SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 62:65–74. doi:10.1124/mol.62.1.65
Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, Manova-Todorova K, Blasberg R, Gerald WL, Massagué J (2005) Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci USA 102:13909–13914. doi:10.1073/pnas.0506517102
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174. doi:10.1038/nrc745
van’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–536. doi:10.1038/415530a
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J (2005) Genes that mediate breast cancer metastasis to lung. Nature 436:518–524. doi:10.1038/nature03799
Tamura Y, Watanabe F, Nakatani T, Yasui K, Fuji M, Komurasaki T, Tsuzuki H, Maekawa R, Yoshioka T, Kawada K, Sugita K, Ohtani M (1998) Highly selective and orally active inhibitors of type IV collagenase (MMP-9 and MMP-2): N-sulfonylamino acid derivatives. J Med Chem 41:640–649. doi:10.1021/jm9707582
Cowell JK, Laduca J, Rossi MR, Burkhardt T, Nowak NJ, Matsui S (2005) Molecular characterization of the t(3;9) associated with immortalization in the MCF10A cell line. Cancer Genet Cytogenet 163:23–29. doi:10.1016/j.cancergencyto.2005.04.019
Kadota M, Yang HH, Gomez B, Sato M, Clifford RJ, Meerzaman D, Dunn BK, Wakefield LM, Lee MP (2010) Delineating genetic alterations for tumor progression in the MCF10A series of breast cancer cell lines. PLoS One 5:e9201. doi:10.1371/journal.pone.0009201
Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ (2008) Collagen density promotes mammary tumor initiation and progression. BMC Med 6:11. doi:10.1186/1741-7015-6-11
Ramaswamy S, Ross KN, Lander ES, Golub TR (2003) A molecular signature of metastasis in primary solid tumors. Nat Genet 33:49–54. doi:10.1038/ng1060
Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ (1992) Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA 89:9064–9068
Debnath J, Muthuswamy SK, Brugge JS (2003) Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30:256–268. doi:10.1016/S1046-2023(03)00032-X
Li Q, Mullins SR, Sloane BF, Mattingly RR (2008) p21-Activated kinase 1 coordinates aberrant cell survival and pericellular proteolysis in a three-dimensional culture model for premalignant progression of human breast cancer. Neoplasia 10:314–329. doi:10.1593/neo.07970
Li Q, Chow AB, Mattingly RR (2010) Three-dimensional overlay culture models of human breast cancer reveal a critical sensitivity to mitogen-activated protein kinase kinase inhibitors. J Pharmacol Exp Ther 332:821–828. doi:10.1124/jpet.109.160390
Garamszegi N, Garamszegi SP, Samavarchi-Tehrani P, Walford E, Schneiderbauer MM, Wrana JL, Scully SP (2010) Extracellular matrix-induced transforming growth factor-beta receptor signaling dynamics. Oncogene 29:2368–2380. doi:10.1038/onc.2009.514
Incorvaia L, Badalamenti G, Rini G, Arcara C, Fricano S, Sferrazza C, Di TD, Gebbia N, Leto G (2007) MMP-2, MMP-9 and activin A blood levels in patients with breast cancer or prostate cancer metastatic to the bone. Anticancer Res 27:1519–1525
Strizzi L, Postovit LM, Margaryan NV, Seftor EA, Abbott DE, Seftor RE, Salomon DS, Hendrix MJ (2008) Emerging roles of nodal and Cripto-1: from embryogenesis to breast cancer progression. Breast Dis 29:91–103
Adkins HB, Bianco C, Schiffer SG, Rayhorn P, Zafari M, Cheung AE, Orozco O, Olson D, De LA, Chen LL, Miatkowski K, Benjamin C, Normanno N, Williams KP, Jarpe M, LePage D, Salomon D, Sanicola M (2003) Antibody blockade of the Cripto CFC domain suppresses tumor cell growth in vivo. J Clin Invest 112:575–587. doi:10.1172/JCI17788
Dzwonek J, Preobrazhenska O, Cazzola S, Conidi A, Schellens A, van DM, Stubbs A, Klippel A, Huylebroeck D, ten Dijke P, Verschueren K (2009) Smad3 is a key nonredundant mediator of transforming growth factor β signaling in Nme mouse mammary epithelial cells. Mol Cancer Res 7:1342–1353. doi:10.1158/1541-7786.MCR-08-0558
Tian F, Byfield SD, Parks WT, Stuelten CH, Nemani D, Zhang YE, Roberts AB (2004) Smad-binding defective mutant of transforming growth factor β type I receptor enhances tumorigenesis but suppresses metastasis of breast cancer cell lines. Cancer Res 64:4523–4530. doi:10.1158/0008-5472.CAN-04-0030
Tian F, DaCosta BS, Parks WT, Yoo S, Felici A, Tang B, Piek E, Wakefield LM, Roberts AB (2003) Reduction in Smad2/3 signaling enhances tumorigenesis but suppresses metastasis of breast cancer cell lines. Cancer Res 63:8284–8292
Stetler-Stevenson WG (1994) Progelatinase A activation during tumor cell invasion. Invasion Metastasis 14:259–268
Coussens LM, Tinkle CL, Hanahan D, Werb Z (2000) MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103:481–490. doi:10.1016/S0092-8674(00)00139-2
Safina A, Vandette E, Bakin AV (2007) ALK5 promotes tumor angiogenesis by upregulating matrix metalloproteinase-9 in tumor cells. Oncogene 26:2407–2422. doi:10.1038/sj.onc.1210046
Tester AM, Waltham M, Oh SJ, Bae SN, Bills MM, Walker EC, Kern FG, Stetler-Stevenson WG, Lippman ME, Thompson EW (2004) Pro-matrix metalloproteinase-2 transfection increases orthotopic primary growth and experimental metastasis of MDA-MB-231 human breast cancer cells in nude mice. Cancer Res 64:652–658
Acknowledgments
We thank our colleagues, in particular Lukas Hawinkels, for valuable discussion and help during experiments. We are grateful to Niek Henriquez, Petra van Overveld, and Geertje van der Horst for their help to set up the invasion assay. We thank Ed Leof (Mayo Clinic, Minnesota, USA) and Ken Iwata (OSI Pharmaceuticals, New York, USA), for reagents; Fred Miller (Barbara Ann Karmanos Cancer Institute, Detroit, USA) for the cell lines. This study was supported by the Dutch Cancer Society (RUL 2005-3371), European Union Framework 6 grant BRECOSM (5032240) and Tumor Host Genomics (518198), Centre for Biomedical Genetics, Swedish Cancerfonden (09 0773), and Ludwig Institute for Cancer Research.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Eliza Wiercinska, Hildegonda P. H. Naber authors contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wiercinska, E., Naber, H.P.H., Pardali, E. et al. The TGF-β/Smad pathway induces breast cancer cell invasion through the up-regulation of matrix metalloproteinase 2 and 9 in a spheroid invasion model system. Breast Cancer Res Treat 128, 657–666 (2011). https://doi.org/10.1007/s10549-010-1147-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1147-x