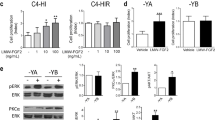
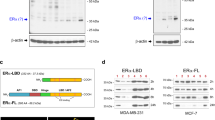
Abstract
Estrogen receptor-α (ERα) positive breast cancer frequently responds to inhibitors of ERα activity, such as tamoxifen, and/or to aromatase inhibitors that block estrogen biosynthesis. However, many patients become resistant to these agents through mechanisms that remain unclear. Previous studies have shown that expression of ERα in ERα-negative breast cancer cell lines frequently inhibits their growth. In order to determine the consequence of ERα over-expression in ERα-positive breast cancer cells, we over-expressed ERα in the MCF-7 breast cancer cell line using adenovirus gene transduction. ERα over-expression led to ligand-independent expression of the estrogen-regulated genes pS2 and PR and growth in the absence of estrogen. Interestingly, prolonged culturing of these cells in estrogen-free conditions led to the outgrowth of cells capable of growth in cultures from ERα transduced, but not in control cultures. From these cultures a line, MLET5, was established which remained ERα-positive, but grew in an estrogen-independent manner. Moreover, MLET5 cells were inhibited by anti-estrogens showing that ERα remains important for their growth. Gene expression microarray analysis comparing MCF-7 cells with MLET5 highlighted apoptosis as a major functional grouping that is altered in MLET5 cells, such that cell survival would be favoured. This conclusion was further substantiated by the demonstration that MLET5 show resistance to etoposide-induced apoptosis. As the gene expression microarray analysis also shows that the apoptosis gene set differentially expressed in MLET5 is enriched for estrogen-regulated genes, our findings suggest that transient over-expression of ERα could lead to increased cell survival and the development of estrogen-independent growth, thereby contributing to resistance to endocrine therapies in breast cancer patients.






Similar content being viewed by others
References
Osborne CK (1998) Tamoxifen in the treatment of breast cancer. N Engl J Med 339(22):1609–1618
Ali S, Coombes RC (2002) Endocrine-responsive breast cancer and strategies for combatting resistance. Nat Rev Cancer 2:101–112
Johnston SR, Dowsett M (2003) Aromatase inhibitors for breast cancer: lessons from the laboratory. Nat Rev Cancer 3(11):821–831
Chen S et al (2006) What do we know about the mechanisms of aromatase inhibitor resistance? J Steroid Biochem Mol Biol 102(1–5):232–240
Kuukasjarvi T et al (1996) Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J Clin Oncol 14(9):2584–2589
Lapidus RG, Nass SJ, Davidson NE (1998) The loss of estrogen and progesterone receptor gene expression in human breast cancer. J Mammary Gland Biol Neoplasia 3(1):85–94
Gee JM et al (2001) Phosphorylation of ERK1/2 mitogen-activated protein kinase is associated with poor response to anti-hormonal therapy and decreased patient survival in clinical breast cancer. Int J Cancer 95(4):247–254
Kirkegaard T et al (2005) AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol 207(2):139–146
Sarwar N et al (2006) Phosphorylation of ERalpha at serine 118 in primary breast cancer and in tamoxifen-resistant tumours is indicative of a complex role for ERalpha phosphorylation in breast cancer progression. Endocr Relat Cancer 13(3):851–861
Osborne CK et al (2003) Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95(5):353–361
Jiang SY, Jordan VC (1992) Growth regulation of estrogen receptor-negative breast cancer cells transfected with complementary DNAs for estrogen receptor. J Natl Cancer Inst 84(8):580–591
Zajchowski DA, Sager R, Webster L (1993) Estrogen inhibits the growth of estrogen receptor-negative but not estrogen receptor-positive, human mammary epithelial cells expressing a recombinant estrogen receptor. Cancer Res 53(20):5004–5011
Lazennec G, Katzenellenbogen BS (1999) Expression of human estrogen receptor using an efficient adenoviral gene delivery system is able to restore hormone-dependent features to estrogen receptor-negative breast carcinoma cells. Mol Cell Endocrinol 149(1–2):93–105
Fowler AM et al (2004) Increases in estrogen receptor-alpha concentration in breast cancer cells promote serine 118/104/106-independent AF-1 transactivation and growth in the absence of estrogen. FASEB J 18(1):81–93
He TC et al (1998) A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95(5):2509–2514
Ali S et al (2009) The development of a selective cyclin-dependent kinase inhibitor that shows antitumor activity. Cancer Res 69(15):6208–6215
Buluwela L et al (2005) Inhibiting estrogen responses in breast cancer cells using a fusion protein encoding estrogen receptor-alpha and the transcriptional repressor PLZF. Gene Therapy 12(5):452–460
Lavinsky RM et al (1998) Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc Natl Acad Sci USA 95(6):2920–2925
Shang Y, Brown M (2002) Molecular determinants for the tissue specificity of SERMs. Science 295(5564):2465–2468
Keeton EK, Brown M (2005) Cell cycle progression stimulated by tamoxifen-bound estrogen receptor-alpha and promoter-specific effects in breast cancer cells deficient in N-CoR and SMRT. Mol Endocrinol 19(6):1543–1554
Hupperets PS et al (1997) The prognostic significance of steroid receptor activity in tumor tissues of patients with primary breast cancer. Am J Clin Oncol 20(6):546–551
Nielsen KV et al (2010) Amplification of ESR1 may predict resistance to adjuvant tamoxifen in postmenopausal patients with hormone receptor positive breast cancer. Breast Cancer Res Treat. doi:10.1007/s10549-010-0984-y
Moelans CB et al (2010) Molecular profiling of invasive breast cancer by multiplex ligation-dependent probe amplification-based copy number analysis of tumor suppressor and oncogenes. Mod Pathol 23(7):1029–1039
Holst F et al (2007) Estrogen receptor alpha (ESR1) gene amplification is frequent in breast cancer. Nat Genet 39(5):655–660
Tomita S et al (2009) Estrogen receptor alpha gene ESR1 amplification may predict endocrine therapy responsiveness in breast cancer patients. Cancer Sci 100(6):1012–1017
Albertson DG (2008) Conflicting evidence on the frequency of ESR1 amplification in breast cancer. Nat Genet 40(7):821–822
Anderson H et al (2007) Predictors of response to aromatase inhibitors. J Steroid Biochem Mol Biol 106(1–5):49–54
Chan CM et al (2002) Molecular changes associated with the acquisition of oestrogen hypersensitivity in MCF-7 breast cancer cells on long-term oestrogen deprivation. J Steroid Biochem Mol Biol 81(4–5):333–341
Lannigan DA (2003) Estrogen receptor phosphorylation. Steroids 68:1–9
Brunner N et al (1993) Acquisition of hormone-independent growth in MCF-7 cells is accompanied by increased expression of estrogen-regulated genes but without detectable DNA amplifications. Cancer Res 53(2):283–290
Naughton C et al (2007) Progressive loss of estrogen receptor alpha cofactor recruitment in endocrine resistance. Mol Endocrinol 21(11):2615–2626
Zhang J et al (1998) Proteasomal regulation of nuclear receptor corepressor-mediated repression. Genes Dev 12(12):1775–1780
Frasor J et al (2005) Estrogen down-regulation of the corepressor N-CoR: mechanism and implications for estrogen derepression of N-CoR-regulated genes. Proc Natl Acad Sci USA 102(37):13153–13157
Shou J et al (2004) Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96(12):926–935
Kirkegaard T et al (2007) Amplified in breast cancer 1 in human epidermal growth factor receptor—positive tumors of tamoxifen-treated breast cancer patients. Clin Cancer Res 13(5):1405–1411
Martin LA et al (2003) Enhanced estrogen receptor (ER) alpha, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term estrogen deprivation. J Biol Chem 278(33):30458–30468
Santen RJ, et al (2005) Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity. Endocr Relat Cancer 12(Suppl 1): S61–S73
Song RX et al (2005) Down-regulation of Bcl-2 enhances estrogen apoptotic action in long-term estradiol-depleted ER(+) breast cancer cells. Apoptosis 10(3):667–678
Callagy GM et al (2006) Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res 12(8):2468–2475
Cannings E et al (2007) Bad expression predicts outcome in patients treated with tamoxifen. Breast Cancer Res Treat 102(2):173–179
Coser KR et al (2009) Antiestrogen-resistant subclones of MCF-7 human breast cancer cells are derived from a common monoclonal drug-resistant progenitor. Proc Natl Acad Sci USA 106(34):14536–14541
Acknowledgments
We would like to thank Georges Vassaux and Nick Lemoine for facilitating the generation of the adenoviruses used in this study. This work was made possible by grants from Cancer Research UK and the Breast Cancer Research Trust. We are grateful for support from the NIHR Biomedical Research Centre funding scheme. We also thank the CR-UK and the Dept of Health funded Imperial College Experimental Cancer Medicine Centre (ECMC).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Robert S. Tolhurst and Ross S. Thomas should be considered as joint first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tolhurst, R.S., Thomas, R.S., Kyle, F.J. et al. Transient over-expression of estrogen receptor-α in breast cancer cells promotes cell survival and estrogen-independent growth. Breast Cancer Res Treat 128, 357–368 (2011). https://doi.org/10.1007/s10549-010-1122-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1122-6