Abstract
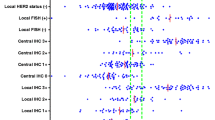
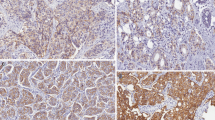
This retrospective study investigates the correlation of intra-individual HER2 status between primary breast cancers and corresponding recurrences in a population derived cohort. The REMARK criteria were used as reference. In 151 breast cancer patients, primary tumors were analyzed for HER2 status on histopathology sections using immunohistochemistry (IHC) confirmed by fluorescence in situ hybridization (FISH) for IHC 2+ and 3+. Recurrences (loco regional and distant) were investigated by aspiration cytology, using HER2 immunocytochemistry (ICC) or FISH (ICC in 84 patients and FISH in 102 patients). In the 151 patients, sites of recurrence were bone/bone marrow 30%, liver 16%, local recurrence 18%, lung/pleura 10%, axillary lymph nodes 9%, skin (non-local) 7%, supra clavicular lymph nodes 5%, and other sites 7%. In 15 patients (10%) HER2 status changed, 7 of 108 patients (6%) from HER2 negative to HER2 positive and 8 of 43 (19%) from HER2 positive to HER2 negative. Intra-patient agreement in HER2 status was 76% (95% CI 64–87%), and the disagreement was 10% (95% CI 5–15%). The multivariable Cox analysis showed a significantly increased risk of dying in the patient group with changed HER2 status compared to patients with concordant positive HER2 status. Overall survival HR is 5.47 (95% CI 2.01–14.91) and survival from relapse HR is 3.22 (95% CI 1.18–8.77). The unstable status for HER2 in breast cancer is clinically significant and should motivate more frequent testing of recurrences.



Similar content being viewed by others
References
Ross SJ, Fletcher JA, Linette GP (2003) The HER-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist 8:307–325
Slamon DJ, Clark GM, Wong SG et al (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER2/neu oncogene. Science 235(4785):177–182
Slamon DJ, Leyland-Jones B, Shalk S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2/neu/NEU for metastatic breast cancer that overexpresses HER2/neu/NEU. N Engl J Med 44(11):783–792
Marty M, Cognetti F, Maraninchi D et al (2001) Randomised phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor-2 positive metastatic breast cancer administered as first line treatment: the M77001 study group. J Clin Oncol 9: 4265–4274
Slamon D, Eiermann W, Robert N, Pienowski T, Martin M, Rolski J et al (2009) Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC → T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC → TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients: BCIRG 006 study. SABCS (abstract 62)
Ryden L, Haglund M, Bendahl P et al (2009) Reproducibility of human epidermal growth factor receptor 2 analysis in primary breast cancer—a national survey performed at pathology departments in Sweden. Acta Oncol 48:860–866
MacFarlane R, Speers C, Masoudi H et al (2008) Molecular change in the primary breast cancer versus the relapsed/metastatic lesion from a large population based database and tissue micro array series. Proc Am Soc Clin Oncol 44:26 (abstract 1000)
Lower EE, Glass E (2009) Blau B HER-2/neu expression in primary and metastatic breast cancer. Breast Cancer Res Treat 113:301–306
Liedtke C, Broglio K, Moulder S et al (2009) Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol (e-print)
Meng S, Tripathy D, Shete S et al (2004) HER-2 gene amplification can be acquired as breast cancer progresses. PNAS 101(25):9393–9398
Yaziji H, Goldstein LC, Barry TS et al (2004) HER2 amplification ratios in breast cancer using parallel tissue based methods. JAMA 291:1972–1977
McShane L, Altman D, Sauerbrei W et al (2005) Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 23(36):9067–9072
Landis JR, Koch GG (1977) An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 33(2):363–374
Schoenfeld D (1982) Partial residuals for the proportional hazards regression model. Biometrika 69:239–241
Mittendorf E, Wu Y, Scaltriti M et al (2009) Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res 15(23):7381–7388
Fehm T, Gebauer G, Jäger W (2002) Clinical utility of serial serum c-erbB-2 determinations in the follow-up of breast cancer patients. Breast Cancer Res Treat 75(2):97–106
Pectasides D, Gaglia A (2006) HER2/neu status of primary breast cancer and corresponding metastatic sites in patients with advanced breast cancer treated with trastuzumab-based therapy. Anticancer Res 26(1B):647–653
Gancberg D, Di Leo A, Cardoso F et al (2002) Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann Oncol 13:1036–1043
Franco A, Col N (2004) Discordance in estrogen (ER) and progestin receptor (PR) status between primary metastatic breast cancer: a meta-analysis. In: Perry M (ed) 40th annual meeting of the ASCO, New Orleans, LA, 11 pp (abstract 539)
Munzone E, Curigliano G, Rocca A et al (2006) Reverting estrogen-receptor-negative phenotype in HER-2-overexpressing advanced breast cancer patients exposed to trastuzumab plus chemotherapy. Breast Cancer Res 8:R4
Lipton A, Leitzel K, Ali SM et al (2005) Serum HER-2/neu conversion to positive at the time of disease progression in patients with breast carcinoma on hormone therapy. Cancer 104(2):257–263
Pauletti G, Danekar RongH et al (2000) Assessment of methods for tissues-based detection of the HER-2/neu alteration in human breast cancer: direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol 18(21):3651–3664
Roche PC, Suman VJ, Jenkins RB et al (2002) Concordance between local and central laboratory HER2 testing in the breast intergroup trial N9831. J Natl Cancer Inst 94(11):855–857
Wolf AC (2007) American society of clinical oncology/college of American pathologists guideline < recommendations for human epidermal growth receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145
Wu JM, Fackler MJ, Halushka MK et al (2008) Heterogeneity of breast cancer metastases: comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res 14(7):1938–1946
Cottu PH, Asselah J, Lae M et al (2008) Intratumoral heterogeneity of HER-2/neu expression and its consequences for the management for advanced breast cancer. Ann Oncol 19:596–597
Andersson J, Linderholm B, Bergh J, Elmberger G (2004) HER-2/neu (c-erbB-2) evaluation in primary breast carcinoma by fluorescent in situ hybridization and immunohistochemistry with special focus on intratumor heterogeneity and comparison of invasive and in situ components. Appl Immunohistochem Mol Morphol 12(1):14–20
Ottesen G, Christensen J, Larsen J et al (2000) Carcinoma in situ of the breast: correlation of histopathology to immunohistochemical markers and DNA ploidy. Breast Cancer Res Treat 60(3):219–226
Norberg T, Klaar S, Kärf G, Nordgren H, Holmberg L, Bergh J (2001) Increased p53 mutation frequency during tumor progression—results from a breast cancer cohort. Cancer Res 61(22):8317–8321
Shah SP, Morin RD, Khattra J et al (2009) Mutational evolution in a lobular breast cancer tumor profiled at single nucleotide resolution. Nature 461:809–913
Acknowledgments
Jan Adolfsson and Ulla Johansson were supported by The Stockholm Regional Oncologic Centre. Nils Wilking was supported by The Local Breast Cancer Tumor Bank, Torsten Hägersten Karolinska Institutet. Jonas Bergh’s research group is supported by the grants from: The Swedish Cancer Society, The Stockholm Cancer Society, The King Gustav V Jubilee Fund, The Swedish Research Council, The Stockholm City Council, Karolinska Institutet and Stockholm County Council Research Strategy Committee, The Swedish Breast Cancer Association (BRO), Märit and Hans Rausing, and The Karolinska Institutet Research Funds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilking, U., Karlsson, E., Skoog, L. et al. HER2 status in a population-derived breast cancer cohort: discordances during tumor progression. Breast Cancer Res Treat 125, 553–561 (2011). https://doi.org/10.1007/s10549-010-1029-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1029-2