Abstract
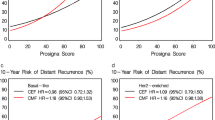
The aim of this study was to examine TOP2A gene copy number changes as a means to identify groups of breast cancer patients with superior benefit from treatment with anthracyclines. Tumour tissue was retrospectively collected and successfully analysed for TOP2A in 773 of 980 Danish patients randomly assigned to receive intravenous CMF (cyclophosphamide, methotrexate and fluorouracil) or CEF (cyclophosphamide, epirubicin and fluorouracil) in DBCG trial 89D. Subgroup analyses on this material published by Knoop et al. (J Clin Oncol 23:7483–7490, 2005) and updated by Nielsen et al. (Acta Oncol 47:725–734, 2008) demonstrated that superiority of CEF over CMF is limited to patients with TOP2A aberrations, defined as patients whose tumours have TOP2A ratio below 0.8 or above 2.0. The Subpopulation Treatment Effect Pattern Plot (STEPP) technique was applied to these data to explore the pattern of treatment effect relative to TOP2A and to compare that pattern to the ranges previously used to define ‘aberrations’. The pattern of treatment effect illustrated by the STEPP analysis confirmed that the superiority of CEF over CMF is indeed limited to patients whose tumours have high or low TOP2A ratios. The hypothesis of no treatment effect–covariate interaction was rejected (P = 0.02). Furthermore, results indicated that the interval of TOP2A ratios hitherto denoted as ‘normal’ could be narrower than previously assumed. A more optimal separation of TOP2A subgroups could be obtained by altering cut-points currently used to define TOP2A amplified and TOP2A deleted tumours by narrowing the TOP2A normal interval, and consequently enlarging the population with TOP2A aberrated tumours.



Similar content being viewed by others
References
Gudkov AV, Zelnick CR, Kazarov AR, Thimmapaya R, Suttle DP, Beck WT, Roninson IB (1993) Isolation of genetic suppressor elements, inducing resistance to topoisomerase II-interactive cytotoxic drugs, from human topoisomerase II cDNA. Proc Natl Acad Sci USA 90:3231–3235
Ejlertsen B, Mouridsen HT, Jensen MB, Andersen J, Cold S, Edlund P, Ewertz M, Jensen BB, Kamby C, Nordenskjold B, Bergh J (2007) Improved outcome from substituting methotrexate with epirubicin: results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur J Cancer 43:877–884
Coombes RC, Bliss JM, Wils J, Morvan F, Espie M, Amadori D, Gambrosier P, Richards M, Aapro M, Villar-Grimalt A, McArdle C, Perez-Lopez FR, Vassilopoulos P, Ferreira EP, Chilvers CE, Coombes G, Woods EM, Marty M (1996) Adjuvant cyclophosphamide, methotrexate, and fluorouracil versus fluorouracil, epirubicin, and cyclophosphamide chemotherapy in premenopausal women with axillary node-positive operable breast cancer: results of a randomized trial. The International Collaborative Cancer Group. J Clin Oncol 14:35–45
Levine MN, Bramwell VH, Pritchard KI, Norris BD, Shepherd LE, Abu-Zahra H, Findlay B, Warr D, Bowman D, Myles J, Arnold A, Vandenberg T, MacKenzie R, Robert J, Ottaway J, Burnell M, Williams CK, Tu D (1998) Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 16:2651–2658
Carpenter JT, Velez-Garcia E, Aron BS et al (1991) Five year results of a randomized comparison of cyclophosphamide, doxorubicin and fluorouracil (CAF) versus cyclophosphamide, methotrexate and fluorouracil (CMF) for node positive breast cancer: a Southeastern Cancer Group Study. Proc Am Soc Clin Oncol 13:66 (abstr 68)
Martin M, Villar A, Sole-Calvo A, Gonzalez R, Massuti B, Lizon J, Camps C, Carrato A, Casado A, Candel MT, Albanell J, Aranda J, Munarriz B, Campbell J, Diaz-Rubio E (2003) Doxorubicin in combination with fluorouracil and cyclophosphamide (i.v. FAC regimen, day 1, 21) versus methotrexate in combination with fluorouracil and cyclophosphamide (i.v. CMF regimen, day 1, 21) as adjuvant chemotherapy for operable breast cancer: a study by the GEICAM group. Ann Oncol 14:833–842
Hutchins LF, Green SJ, Ravdin PM, Lew D, Martino S, Abeloff M, Lyss AP, Allred C, Rivkin SE, Osborne CK (2005) Randomized, controlled trial of cyclophosphamide, methotrexate, and fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil with and without tamoxifen for high-risk, node-negative breast cancer: treatment results of Intergroup Protocol INT-0102. J Clin Oncol 23:8313–8321
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
Poole CJ, Earl HM, Hiller L et al (2006) Epirubicin and cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy for early breast cancer. N Engl J Med 355:1851–1862
Fisher B, Brown AM, Dimitrov NV et al (1990) Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol 8:1483–1496
Knoop AS, Knudsen H, Balslev E, Rasmussen BB, Overgaard J, Nielsen KV, Schonau A, Gunnarsdóttir KA, Olsen KE, Mouridsen H, Ejlertsen B (2005) Retrospective analysis of topoisomerase IIα amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, epirubicin and fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol 23:7483–7490
O’Malley FP, Chia S, Tu D, Shepherd LE, Levine MN, Bramwell VH, Andrulis IL, Pritchard KI (2009) Topoisomerase II alpha and responsiveness of breast cancer to adjuvant chemotherapy. J Natl Cancer Inst 101:644–650
Nielsen KV, Ejlertsen B, Møller S, Jørgensen JT, Knoop A, Knudsen H, Mouridsen HT (2008) The value of TOP2A gene copy number variation as a biomarker in breast cancer: update of DBCG trial 89D. Acta Oncol 47:725–734
Harris LN, Broadwater G, Abu-Khalaf M et al (2009) Topoisomerase IIα amplification does not predict benefit from dose intense cyclophosphamide, doxorubicin, and fluorouracil therapy in HER2-amplified early breast cancer: results of CALGB 8541/150013. J Clin Oncol 27:3430–3436
Tubbs RR, Barlow WE, Budd GT, Swain E et al (2009) Outcome of patients with early-stage breast cancer treated with doxorubicin-based adjuvant chemotherapy as a function of HER2 and TOP2A status. J Clin Oncol 27:3881–3886
Järvinen TAH, Tanner M, Rantanen V, Bärlund M, Borg Å, Grénman S, Isola J (2000) Amplification and deletion of topoisomerase IIα associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. Am J Pathol 156:839–847
Olsen KE, Knudsen H, Rasmussen BB, Balslev E, Knoop A, Ejlertsen B, Nielsen KV, Schönau A, Overgaard J (2004) Amplification of HER2 and TOP2A and deletion of TOP2A genes in breast cancer investigated by new FISH probes. Acta Oncol 43:35–42
Di Leo A, Larsimont D, Gancberg D, Tanner M, Jarvinen T, Rouas G, Dolci S, Leroy J, Paesmans M, Isola J, Piccart M (2002) HER-2 amplification and topoisomerase IIα gene aberrations as predictive markers in node-positive breast cancer patients randomly treated either with an anthracycline-based therapy or with cyclophosphamide, methotrexate and 5-fluorouracil. Clin Cancer Res 8:1107–1116
Bartlett JMS, Munro A, Cameron DA, Thomas J, Prescott R, Twelves C (2008) Type 1 receptor tyrosine kinase profiles identify patients with enhanced benefit from anthracyclines in the BR9601 Adjuvant Breast Cancer Chemotherapy Trial. J Clin Oncol 26:5027–5035
Bonetti M, Gelber R (2000) A graphical method to assess treatment-covariate interactions using the Cox model on subsets of the data. Stat Med 19:2595–2609
Bonetti M, Gelber R (2004) Patterns of treatment effects in subsets of patients in clinical trials. Biostatistics 5:465–481
Bonetti M, Zahrieh D, Cole BF, Gelber RD (2009) A small sample study of the STEPP approach to assessing treatment-covariate interactions in survival data. Stat Med 28:1255–1268
Bartlett JMS, Munro AF, Dunn JA, McConkey C et al (2010) Predictive markers of anthracycline benefit: a prospectively planned analysis of the UK National Epirubicin Adjuvant Trial (NEAT/BR9601). Lancet Oncol 11:266–274
Tanner M, Isola J, Wiklund T, Erikstein B, Kellokumpu-Lehtinen P, Malmström P, Wilking N, Nilsson J, Bergh J (2006) Topoisomerase IIα gene amplification predicts favourable treatment response to tailored and dose-escalated anthracycline based adjuvant chemotherapy in HER-2/neu-amplified breast cancer: Scandinavian Breast Group Trial 9401. J Clin Oncol 24:2428–2436
Acknowledgements
This study was supported in part by Grant No. CA-75362 from the United States National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunnarsdóttir, K.Á., Jensen, MB., Zahrieh, D. et al. CEF is superior to CMF for tumours with TOP2A aberrations: a Subpopulation Treatment Effect Pattern Plot (STEPP) analysis on Danish Breast Cancer Cooperative Group Study 89D. Breast Cancer Res Treat 123, 163–169 (2010). https://doi.org/10.1007/s10549-010-0931-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0931-y